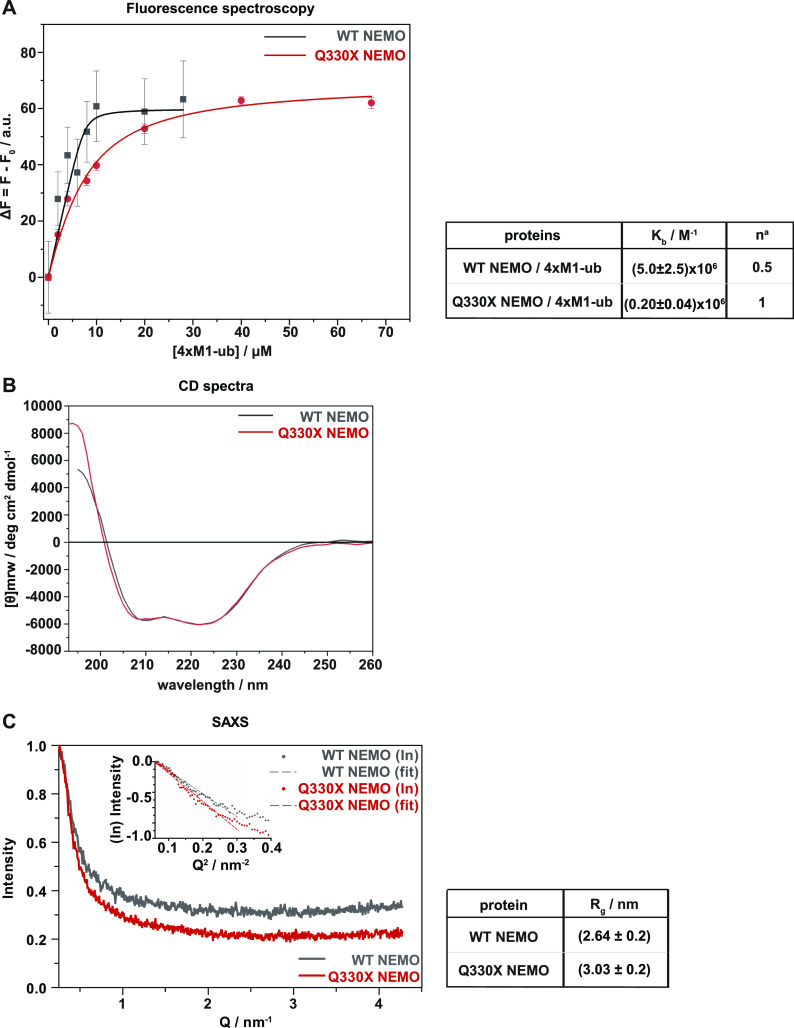
Figure 5. Binding of Q330X NEMO to M1-linked ubiquitin is impaired.
(A) Q330X NEMO shows a reduced binding affinity for 4×M1-ub. Binding isotherms obtained by steady-state fluorescence spectroscopy for the complex formation between untagged WT NEMO (black squares) or Q330X NEMO (red circles) and recombinant 4×M1-ub at 25°C in 10 mM Tris–HCl buffer, pH 7.4. The solid lines represent the best fit of experimental data according to an equivalent and independent binding site model. an is the stoichiometry defined as mol of linear tetra-ubiquitin per mol of WT NEMO or Q330X NEMO. (B) WT NEMO and Q330X NEMO show similar, predominantly alpha-helical secondary structures. Circular dichroism spectra were recorded in the Far-UV region (below 260 nm) for WT NEMO and Q330X NEMO at 1 mg/ml concentration. (C) WT NEMO and Q330X NEMO show similar conformations in solution reflected by comparable radius of gyration values obtained by small-angle X-ray scattering. The small-angle X-ray scattering measurements of WT NEMO and mutant Q330X NEMO were made at a concentration of 5 mg/ml in 10 mM Tris, pH 7.4. The data were obtained by the program “PRIMUS” and are shown as a function of Guinier linear plot for intensity versus Q.