This editorial refers to ‘Association between phonocardiography and echocardiography in heart failure patients with preserved ejection fraction’, by H. Luo et al., https://doi.org/10.1093/ehjdh/ztac073.
Introduction
Since the invention of the stethoscope by René Laënnec in 1816, cardiac auscultation has become an essential component of bedside physical examination. It can be perceived as a form of art in which the conclusions of the examiner are heavily dependent on the acuity of hearing, the sense of timing, and the appreciation of the tonal quality of complex transient noises.1 Although most physicians agree that cardiac auscultation is a skill to be mastered and still represents an important source of clinical information, it receives less and less emphasis in both teaching and clinical practice, which is mostly attributable to its limited diagnostic accuracy and the fact that similar information can be acquired using less subjective diagnostic tools. Although the obtained information is highly dependent on the examiner’s expertise, the improper interpretation of auditory information does not emanate entirely from the lack of experience, as even highly trained physicians often disagree about heart sounds.2 This fact exposes certain human auditory limitations, including but not limited to its notorious insensitivity to low frequencies, slow responses to rapidly occurring, brief sonic events, and limited ability to detect certain sounds in the presence of loud sounds or high ambient noise.3
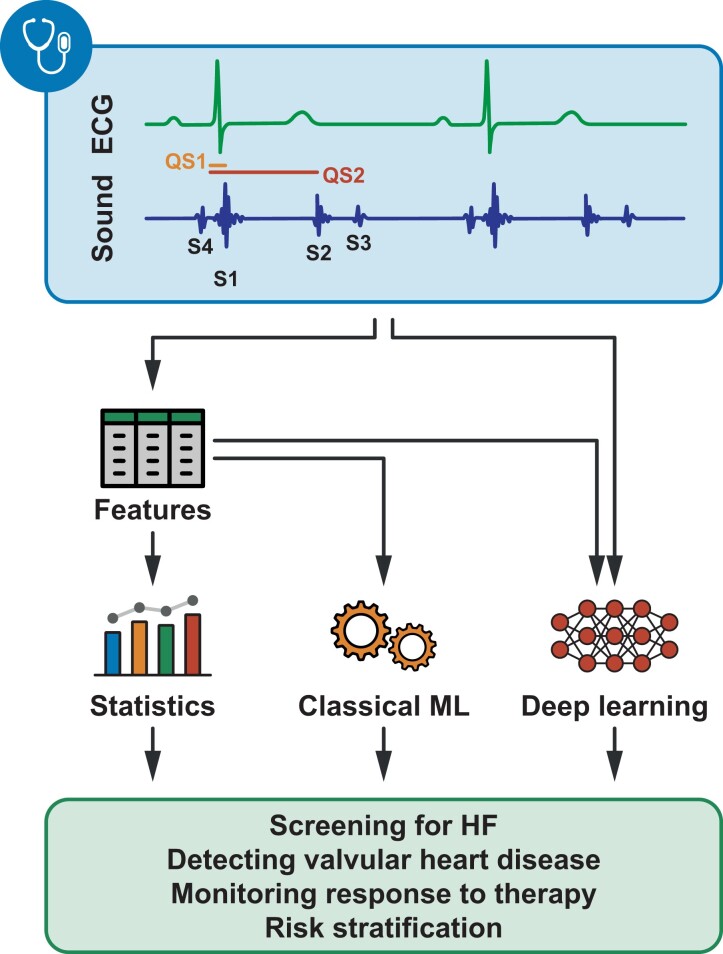
To transcend the aforementioned imperfections of human hearing, it would be highly desirable to capture and record acoustic data graphically that can be analysed free from the subjective distortion of hearing. Motivated by this, phonocardiography was proposed, enabling the detection of abnormal patterns by visual inspection and the extraction of objective features such as amplitudes, frequencies, and time intervals. Phonocardiography not only serves as a vehicle for teaching cardiac auscultation but is also a cost-effective method to identify patients who already have or are at risk of left ventricular (LV) dysfunction, myocardial ischaemia, valvular heart diseases, or congenital heart disease.4,5 Heart sound recordings can also be synchronized with electrical signal tracings of an electrocardiogram, and several additional parameters can be computed that enable the comprehensive and simultaneous assessment of the mechanical and electronic function of the heart. This technique is referred to as acoustic cardiography and has recently attracted increasing attention in heart failure (HF) research (Figure 1).4,6
Figure 1.
Potential applications of acoustic cardiography in heart failure. Multiple approaches exist that can harness the information encompassed by acoustic cardiography recordings. Features (e.g. amplitudes, frequencies, and time intervals) can be extracted manually and analysed using conventional statistics or supplied to classical machine learning or deep learning models. Recordings can also be analysed without manual feature extraction using deep learning. Of note, only a few examples are provided in the figure, and acoustic cardiography might be used for several other tasks within or even outside the realm of heart failure. ECG, electrocardiogram; HF, heart failure; ML, machine learning.
In the present issue of the European Heart Journal – Digital Health, Luo et al.7 presented a pilot study investigating the relationship between acoustic cardiography-derived features and echocardiography-derived E/e′ in patients with suspected HF and preserved ejection fraction (HFpEF). To reduce confounding, the authors performed variable matching with replacement, which resulted in 32 pairs with similar sex, body mass index, and heart rate but different E/e′ values. In each pair, one of the patients was assigned to the low E/e′ group and the other to the high E/e′ group. Due to the inherent nature of the applied matching procedure, the 32 pairs comprised only 25 unique patients, and the same patient might have been assigned to the low E/e′ group in one pair while to the high E/e′ group in another pair. In the high E/e′ group, higher S1, S2, and S4 frequencies, more frequent occurrence of S4, and longer QS2 and QS2c were observed. Based on receiver operating characteristic (ROC) analysis, QS1 was identified as the best marker of E/e′ higher than 9 [0.72, 95% confidence interval (CI): 0.51 – 0.88] and exhibited an area under the ROC curve (AUC) similar to N-terminal pro-brain natriuretic peptide (0.67, 95% CI: 0.46 – 0.85).
Discussion
Luo et al.’s study7 exemplifies how acoustic cardiography could be used to detect elevated LV filling pressures in patients with suspected HFpEF. By providing acoustic biomarkers in a non-invasive and cost-efficient fashion, acoustic cardiography can serve as a screening tool to facilitate HF diagnosis, especially when echocardiography and other advanced diagnostic tools are out of reach (Table 1). Thus, studies accumulating evidence on the potential role of acoustic cardiography in this patient population may have a high clinical impact.
Table 1.
Comparison of cardiac auscultation, acoustic cardiography, and echocardiography
| Cardiac auscultation | Acoustic cardiography | Echocardiography | |
|---|---|---|---|
| Device cost | $ | $$ | $$$$ |
| Required level of experience | ↑↑↑ | ↑ | ↑↑↑↑ |
| Diagnostic accuracy | ↓ | ↑ | ↑↑↑↑ |
| Interobserver agreement | ↓↓ | ↑↑ | ↑↑↑ |
| Established prognostic value | ↑↑ | ↑↑ | ↑↑↑↑ |
| Option to save data | No | Yes | Yes |
| Use in clinical practice | ↑↑↑ | ↓ | ↑↑↑↑ |
Another appealing aspect of acoustic cardiography is that the reported alterations in acoustic features can be directly linked to pathophysiological processes characteristic of HF, such as frequencies of S1 are directly proportional to LV elasticity and inversely proportional to LV mass,8 the prolongation of QS1 implies slower myocardial force development and elevated atrial pressure, and an audible S4 usually indicates a forceful left atrial contraction combined with reduced LV compliance and hence provides a direct clue of diastolic dysfunction.9,10 Thus, acoustic cardiography has a true potential to detect not only LV systolic dysfunction11,12 but also Doppler-based13 and invasively measured elevated filling pressures.12,14 Nevertheless, the diagnostic power of most parameters is still far from perfect.12
Likewise, the results of the current study should be taken with a grain of salt. First, given the single-centre design and the small sample size, the findings should be confirmed in larger, preferably multi-centre studies. These studies would also provide an opportunity to investigate the prognostic value of the proposed acoustic biomarkers. Second, the AUCs of the individual features are rather low and have wide confidence intervals with lower bounds often below 0.5, implying that not a single parameter but rather a combination of multiple features should be used to estimate E/e′. Nevertheless, constructing multivariable models was not possible due to the low number of patients enrolled. Third, it should also be noted that high E/e′ values might not necessarily indicate backward failure in this patient cohort as the correlation of E/e′ with invasively measured filling pressures is rather modest in HFpEF patients.15 Therefore, the associations between the proposed biomarkers and the invasively measured filling pressures should be investigated in future studies.
Despite the intriguing results of recent studies, technical shortcomings of acoustic cardiography should also be acknowledged, including its inability to differentiate between separate frequencies of various sounds, the frequent presence of endogenous and exogenous noises and artefacts that may visually mask weak heart sounds, and the difficulty of identifying the exact onset, peak, and end of heart sounds. In theory, machine learning (ML) is well suited to tackle these technical challenges and has the potential to tap into diagnostic capabilities unreachable with the conventional interpretation of acoustic signals.6 Either using manually extracted heart sound features or automating feature extraction with deep learning techniques, ML can facilitate the identification of patients with HFpEF and HFrEF,16,17 may perform HF stage classification,18 and may be used for several tasks within or even outside the realm of HF, such as for the detection and grading of valvular heart disease19 or screening for congenital heart disease in paediatric patients.20 Although there is little doubt that ML will increase the diagnostic accuracy of acoustic cardiography, there is still a long way to go before such tools will permeate clinical care.
In summary, acoustic biomarkers have great potential in the cost-efficient and prompt identification of patients with HFpEF. Thus, the time has not come to discard the stethoscope, but this time-honoured examination technique should keep pace with more advanced modalities by adopting the latest technological advancements.
Contributor Information
Márton Tokodi, Division of Cardiovascular Diseases and Hypertension, Rutgers Robert Wood Johnson Medical School, 125 Paterson Street, New Brunswick, NJ 08091, USA; Heart and Vascular Centre, Semmelweis University, 68 Városmajor Street, Budapest 1122, Hungary.
Attila Kovács, Heart and Vascular Centre, Semmelweis University, 68 Városmajor Street, Budapest 1122, Hungary.
Funding
Project no. RRF-2.3.1-21-2022-00004 (MILAB) has been implemented with the support provided by the European Union. A.K. was also supported by a grant from the National Research, Development, and Innovation Office (NKFIH) of Hungary (FK 142573).
References
- 1. Sprague HB, Ongley PA. The clinical value of phonocardiography. Circulation 1954;9:127–134. [DOI] [PubMed] [Google Scholar]
- 2. Lok CE, Morgan CD, Ranganathan N. The accuracy and interobserver agreement in detecting the ‘gallop sounds’ by cardiac auscultation. Chest 1998;114:1283–1288. [DOI] [PubMed] [Google Scholar]
- 3. Selig MB. Stethoscopic and phonoaudio devices: historical and future perspectives. Am Heart J 1993;126:262–268. [DOI] [PubMed] [Google Scholar]
- 4. Wen YN, Lee AP, Fang F, Jin CN, Yu CM. Beyond auscultation: acoustic cardiography in clinical practice. Int J Cardiol 2014;172:548–560. [DOI] [PubMed] [Google Scholar]
- 5. Tavel ME. Cardiac auscultation: a glorious past—and it does have a future! Circulation 2006;113:1255–1259. [DOI] [PubMed] [Google Scholar]
- 6. Nahar JK, Lopez-Jimenez F. Utilizing conversational artificial intelligence, voice, and phonocardiography analytics in heart failure care. Heart Fail Clin 2022;18:311–323. [DOI] [PubMed] [Google Scholar]
- 7. Luo H, Weerts J, Bekkers A, Achten A, Lievens S, Smeets K, et al. . Association between phonocardiography and echocardiography in heart failure patients with preserved ejection fraction. Eur Heart J Digital Health 2023;4:4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Adolph RJ, Stephens JF, Tanaka K. The clinical value of frequency analysis of the first heart sound in myocardial infarction. Circulation 1970;41:1003–1014. [DOI] [PubMed] [Google Scholar]
- 9. Shah PM, Gramiak R, Kramer DH, Yu PN. Determinants of atrial (S4) and ventricular (S3) gallop sounds in primary myocardial disease. N Engl J Med 1968;278:753–758. [DOI] [PubMed] [Google Scholar]
- 10. Homma S, Bhattacharjee D, Gopal A, Correia J. Relationship of auscultatory fourth heart sound to the quantitated left atrial filling fraction. Clin Cardiol 1991;14:671–674. [DOI] [PubMed] [Google Scholar]
- 11. Kosmicki DL, Collins SP, Kontos MC, Zuber M, Kipfer P, Jost CA, et al. . Noninvasive prediction of left ventricular systolic dysfunction in patients with clinically suspected heart failure using acoustic cardiography. Congest Heart Fail 2010;16:249–253. [DOI] [PubMed] [Google Scholar]
- 12. Marcus GM, Gerber IL, McKeown BH, Vessey JC, Jordan MV, Huddleston M, et al. . Association between phonocardiographic third and fourth heart sounds and objective measures of left ventricular function. JAMA 2005;293:2238–2244. [DOI] [PubMed] [Google Scholar]
- 13. Collins SP, Lindsell CJ, Kontos MC, Zuber M, Kipfer P, Jost CA, et al. . Bedside prediction of increased filling pressure using acoustic electrocardiography. Am J Emerg Med 2009;27:397–408. [DOI] [PubMed] [Google Scholar]
- 14. Collins SP, Kontos MC, Michaels AD, Zuber M, Kipfer P, Jost CA, et al. . Utility of a bedside acoustic cardiographic model to predict elevated left ventricular filling pressure. Emerg Med J 2010;27:677–682. [DOI] [PubMed] [Google Scholar]
- 15. Nauta JF, Hummel YM, van der Meer P, Lam CSP, Voors AA, van Melle JP.. Correlation with invasive left ventricular filling pressures and prognostic relevance of the echocardiographic diastolic parameters used in the 2016 ESC heart failure guidelines and in the 2016 ASE/EACVI recommendations: a systematic review in patients with heart failure with preserved ejection fraction. Eur J Heart Fail 2018; 20:1303–1311. [DOI] [PubMed] [Google Scholar]
- 16. Damani DN, Damani S, Kapoor A, Kulkarni K, Shivaram S, Araoz PA, et al. . Abstract 14758: Novel deep learning algorithm to detect heart failure with preserved ejection fraction (HFpEF) using computer aided auscultation of phonocardiogram. Circulation 2022;146:A14758. [Google Scholar]
- 17. Gao S, Zheng Y, Guo X. Gated recurrent unit-based heart sound analysis for heart failure screening. Biomed Eng Online 2020;19:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zheng Y, Guo X, Wang Y, Qin J, Lv F. A multi-scale and multi-domain heart sound feature-based machine learning model for ACC/AHA heart failure stage classification. Physiol Meas 2022;43:065002. [DOI] [PubMed] [Google Scholar]
- 19. Al-Issa Y, Alqudah AM. A lightweight hybrid deep learning system for cardiac valvular disease classification. Sci Rep 2022;12:14297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Burns J, Ganigara M, Dhar A. Application of intelligent phonocardiography in the detection of congenital heart disease in pediatric patients: a narrative review. Prog Pediatr Cardiol 2022;64:101455. [Google Scholar]