Abstract
Aims
Heart failure with preserved ejection fraction (HFpEF) is associated with stiffened myocardium and elevated filling pressure that may be captured by heart sound (HS). We investigated the relationship between phonocardiography (PCG) and echocardiography in symptomatic patients suspected of HFpEF.
Methods and results
Consecutive symptomatic patients with sinus rhythm and left ventricular ejection fraction >45% were enrolled. Echocardiography was performed to evaluate the patients’ diastolic function, accompanied by PCG measurements. Phonocardiography features including HS amplitude, frequency, and timing intervals were calculated, and their abilities to differentiate the ratio between early mitral inflow velocity and early diastolic mitral annular velocity (E/e′) were investigated. Of 45 patients, variable ratio matching was applied to obtain two groups of patients with similar characteristics but different E/e′. Patients with a higher E/e′ showed higher first and second HS frequencies and more fourth HS and longer systolic time intervals. The interval from QRS onset to first HS was the best feature for the prediction of E/e′ > 9 [area under the curve (AUC): 0.72 (0.51–0.88)] in the matched patients. In comparison, N-terminal pro-brain natriuretic peptide (NT-proBNP) showed an AUC of 0.67 (0.46–0.85), a value not better than any PCG feature (P > 0.05).
Conclusion
Phonocardiography features stratify E/e′ in symptomatic patients suspected of HFpEF with a diagnostic performance similar to NT-proBNP. Heart sound may serve as a simple non-invasive tool for evaluating HFpEF patients.
Keywords: Heart sound, Phonocardiography, Echocardiography, Heart failure, Heart failure with preserved ejection fraction
Graphical Abstract
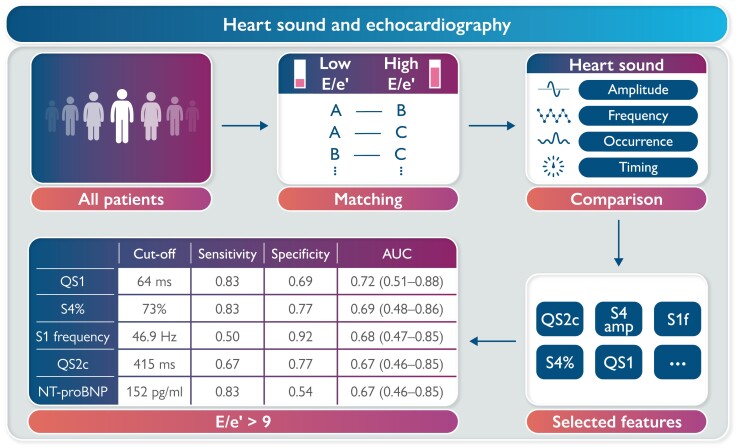
Graphical Abstract.
See the editorial comment for this article ‘Reviving the origins: acoustic biomarkers of heart failure with preserved ejection fraction’, by M. Tokodi and A. Kovács, https://doi.org/10.1093/ehjdh/ztac075.
Introduction
Heart failure with preserved ejection fraction (HFpEF) accounts for ∼50% of heart failure.1 It is associated with ageing, hypertension, and obesity and characterized by an elevated left ventricular (LV) filling pressure.2 Current HFpEF diagnosis mainly relies on echocardiographic parameters such as the ratio between early mitral inflow velocity and early diastolic mitral annular velocity (E/e′) and serological biomarkers such as N-terminal pro-brain natriuretic peptide (NT-proBNP).3E/e′ has been recommended as a sensitive marker of LV filling pressure elevation in current European heart failure guidelines.3 N-terminal pro-brain natriuretic peptide is a highly sensitive but moderately specific marker of heart failure in acute settings.4 Its level is affected by multiple factors such as the patient’s age and kidney function. Neither E/e′ nor NT-proBNP can be used by patients at home, making early recognition of heart failure challenging.
Heart sound (HS) may serve as a simple non-invasive tool for home monitoring of heart failure with the recent emergence of portable digital stethoscopes and wearable acoustic sensing devices.5,6 The relationship between HS and heart failure has been widely investigated in both animal and human studies.7,8 However, these studies have focused on the third HS (S3) in systolic ventricular dysfunction. The changes in HS in diastolic ventricular dysfunction remain incompletely understood. Recently, several studies utilized machine learning to differentiate HFpEF from normal subjects by HS, but no relations between HS and echocardiography have been demonstrated.9–11 Considering the widespread use and importance of echocardiography nowadays, it may be valuable to link HS to echocardiographic parameters such as E/e′ for evaluation of LV filling pressure elevation.
This pilot study explores the association between phonocardiography (PCG) and echocardiography in a patient cohort suspected of HFpEF. We identified the HS features differing between low and high E/e′ patients, and tested their abilities to predict E/e′ > 9.
Methods
Study approval
This prospective observational study was approved by the medical ethics committee of Maastricht University Medical Center+ (MUMC+). Data were collected from consecutive patients suspected of HFpEF and referred to a diagnostic work up including echocardiography in the MUMC+ between January 2020 and May 2021. All study participants provided written consent.
Patient inclusion and exclusion
Patients meeting the following criteria were included: (i) symptoms and/or signs of heart failure such as dyspnoea and lower-extremity oedema, (ii) sinus rhythm, (iii) a preserved LV ejection fraction (LVEF, >45%) and E/e′ ratio evaluated by echocardiography, and (iv) serum NT-proBNP test performed during the hospital visit.
Clinical data were obtained from routine clinical care blinded for HS results (A.A., K.S., and J.W.). All patients underwent a systematic diagnostic work up for HFpEF as described before, including echocardiography and extensive blood analysis.12 The final diagnosis of HFpEF was determined according to the European Society of Cardiology guideline in an expert panel meeting including heart failure cardiologists and echocardiographers.3
Echocardiographic examination
Echocardiographic examinations including two-dimensional measurements, Doppler and tissue Doppler imaging were performed using a Philips iE33 system (Philips Medical Systems, Andover, MA, USA) with the patients in a resting supine position. Left ventricular function and structure, peak mitral inflow velocity (E-wave and A-wave), deceleration time, isovolumic relaxation time, early diastolic mitral annulus velocity (e′) at the septal and lateral aspects, and left atrial volume were assessed according to the American Society of Echocardiography and the European Association of Cardiovascular Imaging recommendations during routine clinical care.13 All stored image data were analysed by experienced sonographers using Philips IntelliSpace Cardiovascular echocardiographic analysis software during routine clinical care. The sonographers were blinded to the patients’ HS data.
Heart sound and electrocardiogram collections
Heart sounds were recorded on the left fourth intercostal space along midclavicular line while the patients were in sitting resting condition with the body leaning forward on the same day of the echocardiography. A digital stethoscope EKO DUO (frequency range: 20–2000 Hz; Eko Devices Inc., USA) was used to simultaneously record HS and single-lead electrocardiogram (ECG) for 15–30 s. The collected data were transferred via Bluetooth from the EKO device to the cloud, from which data could be downloaded for further offline analysis. Heart sounds were collected by researchers (A.B., S.L., A.A., and K.S.) who were blinded to both echocardiographic and PCG features of the patients.
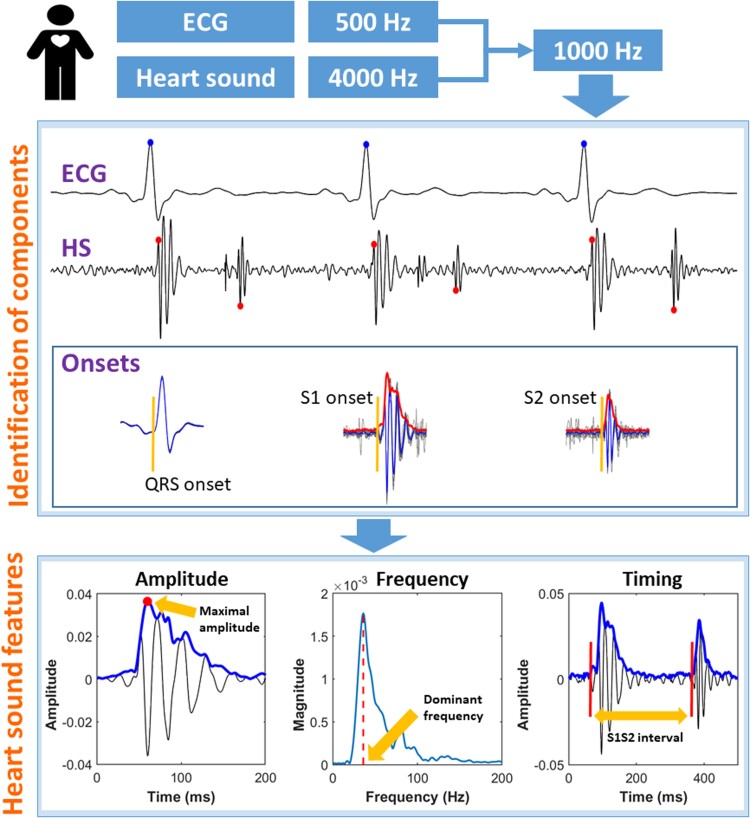
Location of heart sound components
Signal processing procedures are shown in Figure 1 and further described in the Supplementary material online, Methods. In brief, both ECG and PCG recordings were resampled to 1000 Hz. Then ECG was used as a reference to find S1 and S2. To identify the low-frequency S3 and S4, the raw HS recordings were low-pass-filtered with a cut-off frequency of 50 Hz. Occurrences of S3 (S3%) and S4 (S4%) among the 10–15 heartbeats analysed were calculated.
Figure 1.
Demonstration of signal processing procedures. Upper panel: reference points were identified for electrocardiogram QRS (blue dot), S1 (red dot), and S2 (red dot), respectively. Onsets of electrocardiogram QRS, S1, and S2 were identified using a median signal (in blue) calculated from all heartbeats (in grey) aligned at the reference points. Lower panel: multiple heart sound features were calculated. ECG, electrocardiogram; HS, heart sound; S1, first heart sound; S1S2, timing interval from S1 onset to S2 onset; S2, second heart sound.
Heart sound features
For each patient, HS features were calculated from the median value of 10–15 consecutive heartbeats. S1 and S2 features were calculated after low-pass filtering the raw HS signals at 200 Hz, and S3 and S4 at 50 Hz.
Amplitude
Amplitudes of S1 and S2 were automatically identified as the maximal value within 80 ms following their onsets. Amplitudes of S3 and S4 were identified as the maximal value within 60 ms around their respective locations.
Frequency
The dominant frequency was calculated for each HS component. For S1 and S2, a 60 ms segment after their onset was used for fast Fourier transform to avoid potential HS splitting occurring at a later time. For S3 and S4, the onsets were generally difficult to be identified due to the low amplitude; therefore a 60 ms segment around their envelope peaks was used for the calculation of dominant frequency based on a fast Fourier transform.
Timing intervals
The following HS-derived systolic time intervals were analysed: (i) QS1: from the onset of the Q-wave to the onset of S1; (ii) S1S2: from S1 onset to S2 onset; and (iii) QS2: from QRS onset to S2 onset. In analogy to QT, we also applied the Fridericia equation to correct QS2 for heart rate (HR) as follows: QS2c = QS2/RR1/3, in which QS2 and QS2c are in units of milliseconds and RR in seconds.14,15
Statistical analysis
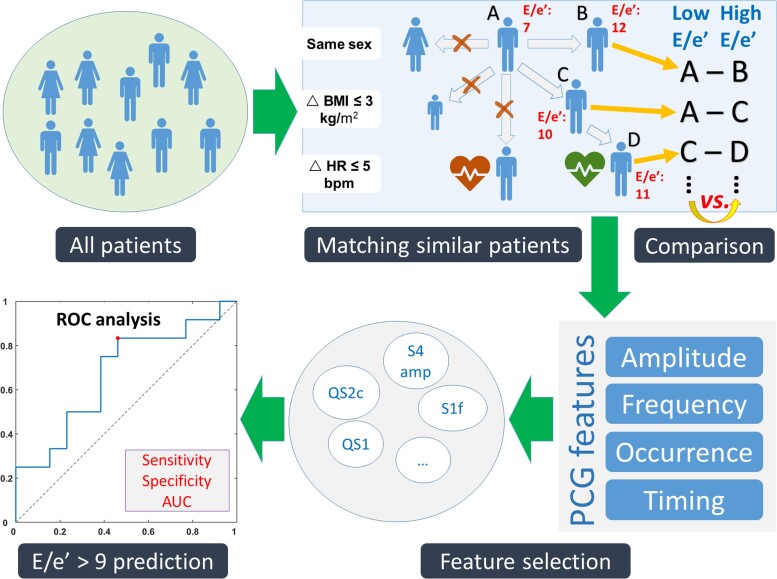
The overarching goal of the statistical analyses was to investigate the relationship between PCG and echocardiography while reducing the effects of confounders as much as possible. To this purpose, a matching procedure was applied as described in the literature.16 The steps are as follows (Figure 2):
Figure 2.
Illustration of statistical analyses. All patients were matched by sex, body mass index, and heart rate and divided into low and high E/e′ groups. Phonocardiographic features were compared between the two groups, and the ones showing a significant difference were identified for the prediction of E/e′ > 9. AUC, area under the curve; BMI, body mass index; E/e′, the ratio between early mitral inflow velocity and early diastolic mitral annular velocity; HR, heart rate; QS1, Q to S1 onset; QS2c, QS2 corrected for RR interval; ROC, receiver operating curve; S1f, S1 dominant frequency; S4, fourth heart sound.
Each patient was matched to the other(s) if both had the same sex and similar body mass indexes (BMIs, difference ≤3 kg/m2) and HRs (difference ≤5 b.p.m.). Doing so, a patient might be matched with multiple patients who satisfied the three conditions above.16 On the other hand, a patient might also not be matched with any other patients and thus be classified into ‘unmatched’ group.
Identification of E/e′-related PCG features: For each match, the two patients were assigned by E/e′ ratio to either low or high E/e′ group. After assigning all patients, the two groups were compared for the PCG features. The features showing a P-value ≤0.10 in the comparison were defined as E/e′-related.
Prediction of E/e′ > 9 using PCG features: The selected PCG features were tested for their performance to predict E/e′ > 9 in the matched patients. The E/e′ cut-off was set at 9 because a higher value at rest generally indicates LV diastolic dysfunction and raised LV filling pressure according to current heart failure guidelines.3 Receiver operating curve analysis was performed, and sensitivity, specificity, and area under the curve (AUC) were calculated. The optimal cut-off value was identified at the maximal Youden’s index (= sensitivity + specificity − 1).17
Continuous and normally distributed data were expressed as mean ± standard deviation and skewed data as median (interquartile range). Count data were expressed as numbers (%). χ2 test was performed to compare the difference in count data between the two groups. Independent sample t-test was used to compare normally distributed continuous variables, while the Wilcoxon rank-sum test was applied for comparison of skewed continuous data. The AUCs and 95% confidence intervals were calculated and compared with the DeLong test using MedCalc (MedCalc Software Ltd, Belgium). Statistical significance was defined as a two-tailed P-value of <0.05. All analyses were performed using MATLAB R2018b (MathWorks Inc., USA).
Results
Baseline characteristics
A total of 61 patients were screened in our study. After excluding 11 patients with atrial fibrillation (AF) during echocardiographic or phonocardiographic measurement, two patients without E/e′ data, one patient without NT-proBNP data, and two patients with too-noisy HS recordings, the remaining 45 patients were matched according to sex, BMI, and HR, resulting in 25 patients that gave rise to 32 matched pairs.
Baseline characteristics of the low and high E/e′ groups did not significantly differ regarding age, sex, BMI, HR, blood pressure, history of hypertension, and history of AF or PR interval (Table 1). One patient had heart failure with recovered LVEF. No patients had prior heart failure hospitalization. The N-terminal pro-brain natriuretic peptide was significantly higher in the high E/e′ group. Significantly more patients were diagnosed with HFpEF in the high than low E/e′ group. Regarding echocardiographic parameters, mean E/e′ was 8.3 and 13.9 in the low and high E/e′ group, respectively. The E/A ratio was similar in both groups.
Table 1.
Population and echocardiographic characteristics
| Low E/e′ | High E/e′ | ||
|---|---|---|---|
| (n = 32) | (n = 32) | P-value | |
| Patient | |||
| Age, years | 72 ± 7 | 74 ± 5 | 0.11 |
| Female, n (%) | 27 (84) | 27 (84) | 1.00 |
| BMI, kg/m2 | 27.5 ± 3.1 | 27.9 ± 3.0 | 0.63 |
| Heart rate, b.p.m. | 71 ± 9 | 71 ± 8 | 0.85 |
| PR interval, ms | 185 ± 31 | 183 ± 26 | 0.75 |
| Systolic BP, mmHg | 146 ± 17 | 150 ± 18 | 0.30 |
| Diastolic BP, mmHg | 78 ± 14 | 76 ± 13 | 0.70 |
| NYHA Class ≥ III, n (%) | 16 (50) | 9 (28) | 0.07 |
| NT-proBNP, pg/mL | 211 ± 172 | 367 ± 291 | 0.01 |
| Hypertension, n (%) | 24 (75) | 20 (63) | 0.28 |
| History of AF, n (%) | 14 (44) | 12 (38) | 0.61 |
| Diabetes, n (%) | 2 (6) | 6 (19) | 0.13 |
| Chronic kidney disease, n (%) | 1 (3) | 5 (16) | 0.09 |
| COPD, n (%) | 4 (13) | 2 (6) | 0.39 |
| HFpEF diagnosis, n (%) | 21 (66) | 30 (94) | 0.005 |
| Echocardiography | |||
| LV mass, g | 123 ± 33 | 158 ± 46 | <0.001 |
| LVMI, g/m2 | 67 ± 14 | 83 ± 17 | <0.001 |
| LAV, mL | 60 ± 20 | 78 ± 19 | <0.001 |
| LAVI, mL/m2 | 33 ± 10 | 41 ± 9 | <0.001 |
| LVEF, % | 58 ± 4 | 62 ± 5 | <0.001 |
| LVEDD, mm | 44 ± 4 | 49 ± 5 | <0.001 |
| LVESD, mm | 30 ± 5 | 31 ± 6 | 0.19 |
| Peak E-wave, cm/s | 66 ± 19 | 87 ± 31 | 0.001 |
| Peak A-wave, cm/s | 63 ± 21 | 83 ± 23 | <0.001 |
| E/A | 1.15 ± 0.59 | 1.15 ± 0.62 | 1.00 |
| e′ lateral, cm/s | 10.0 ± 4.0 | 6.8 ± 2.6 | <0.001 |
| e′ septal, cm/s | 6.5 ± 2.1 | 5.9 ± 1.5 | 0.21 |
| E/e′ average | 8.3 ± 2.2 | 13.9 ± 3.4 | <0.001 |
| E-wave DT, ms | 212 ± 38 | 209 ± 30 | 0.81 |
| A-wave DT, ms | 102 ± 19 | 118 ± 18 | 0.004 |
| IVRT, ms | 96 ± 20 | 114 ± 24 | 0.007 |
AF, atrial fibrillation; A-wave, peak velocity of mitral valve inflow after atrial contraction; BMI, body mass index; BP, blood pressure; COPD, chronic obstructive pulmonary disease; DT, deceleration time; e′, early diastolic mitral annulus velocity by Doppler tissue imaging; E-wave, peak velocity of early diastolic mitral inflow; HFpEF, heart failure with preserved ejection fraction; IVRT, isovolumic relaxation time; LAV, left atrial volume; LAVI, left atrial volume index; LV, left ventricle; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; LVESD, left ventricular end-systolic diameter; LVMI, left ventricular mass index; NT-proBNP, N-terminal pro-brain natriuretic peptide; NYHA, New York Heart Association.
Phonocardiographic characteristics
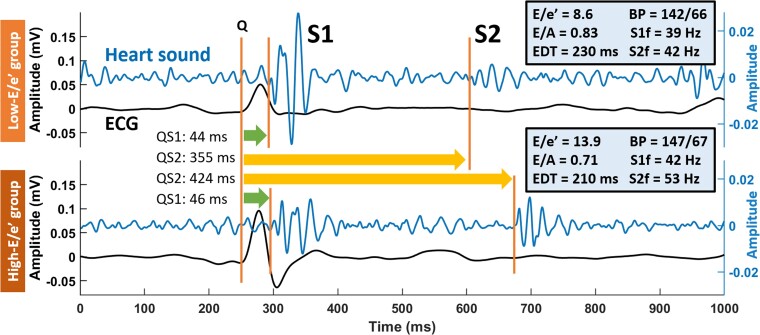
Figure 3 shows examples of HS and ECG recordings from patients of the low and high E/e′ groups. Although QS1 did not markedly differ between low and high E/e′, QS2 was longer in the latter (355 vs. 424 ms), even after correcting for RR interval (QS2c: 405 vs. 461 ms), accompanied by a higher S1 frequency (39 vs. 42 Hz) and S2 frequency (42 vs. 53 Hz) in the high E/e′ patient.
Figure 3.
Examples of electrocardiogram and heart sound in patients with low vs. high E/e′ Both examples were aligned at QRS onset at 250th ms. Note the sharper S1 and S2 morphologies in the lower than the upper panel, indicating higher frequencies at a higher E/e′ ratio. BP, blood pressure in unit of mmHg; ECG, electrocardiogram; EDT, E-wave deceleration time; Q, QRS onset; QS1, Q to S1 onset; QS2, Q to S2 onset; S1, first heart sound; S1f, S1 dominant frequency; S2, second heart sound; S2f, S2 dominant frequency.
Table 2 summarizes the phonocardiographic characteristics of the patients in the two groups. Heart sound amplitude was not significantly different between the two groups, except for a significantly higher S4 amplitude in the high E/e′ group. Frequencies of S1, S2, and S4 were significantly higher in the high than the low E/e′ group. The occurrence of S4 and combined S3 and S4 was higher in the high E/e′ group, but the occurrence of S3 did not differ between the two groups. Regarding timing intervals, the RR interval was nearly identical between the two groups. QS1 tended to be longer in the high E/e′ group (P = 0.10). QS2 and QS2c were longer in the high than low E/e′ group.
Table 2.
Comparison of phonocardiographic features between low and high E/e′
| Low E/e′ | High E/e′ | ||
|---|---|---|---|
| (n = 32) | (n = 32) | P-value | |
| Total heartbeat, n | 410 | 390 | |
| Avg. heartbeat, n | 13 | 12 | |
| Amplitude, ×10−4 | |||
| ȃS1 | 243 (105–322) | 184 (138–220) | 0.14 |
| ȃS2 | 101 (73–151) | 100 (57–224) | 1.00 |
| ȃS3 | 16 (8–24) | 16 (10–23) | 0.99 |
| ȃS4 | 6 (0–20) | 15 (8–21) | 0.03 |
| Frequency | |||
| ȃS1, Hz | 42 ± 3 | 46 ± 8 | 0.003 |
| ȃS2, Hz | 48 ± 6 | 55 ± 14 | 0.008 |
| ȃS3, Hz | 34 (32–34) | 33 (32–34) | 0.17 |
| ȃS4, Hz | 28 (0–34) | 34 (31–34) | 0.02 |
| Occurrence | |||
| ȃS3, % | 93 ± 18 | 91 ± 14 | 0.67 |
| ȃS4, % | 58 ± 34 | 78 ± 29 | 0.01 |
| ȃS3 and S4, % | 54 ± 34 | 71 ± 30 | 0.04 |
| Time interval | |||
| ȃRR, ms | 860 ± 115 | 861 ± 104 | 0.98 |
| ȃQS1, ms | 69 ± 23 | 78 ± 19 | 0.10 |
| ȃS1S2, ms | 320 ± 36 | 333 ± 47 | 0.21 |
| ȃQS2, ms | 389 ± 42 | 412 ± 48 | 0.05 |
| ȃQS2c, ms | 410 ± 33 | 433 ± 41 | 0.01 |
Avg., average; QS1, Q to S1 onset; QS2, Q to S2 onset; QS2c, QS2 corrected for RR interval; S1, first heart sound; S1S2, S1 to S2 onset; S2, second heart sound; S3, third heart sound; S3%, percentage of heartbeats with S3; S3 and S4%, percentage of heartbeats with both S3 and S4; S4, fourth heart sound; S4%, percentage of heartbeats with S4.
Prediction of E/e′ using phonocardiography features
Phonocardiography features that differed between low and high E/e′ groups with a P-value of ≤0.10 in Table 2 were included in E/e′ classification with a cut-off value of 9. Of note, QS2c instead of QS2 was chosen to correct the effect of HR. Table 3 summarizes the optimal cut-off values and diagnostic performance of eligible PCG features and NT-proBNP in predicting E/e′ > 9 in matched patients. The sensitivity was the same (0.83) for all S4-related features including S4 amplitude, S4 frequency, S4% and S3 and S4%, while the specificity was the highest for S4% (0.77). Both S4 amplitude and frequency were dependent on S4% because both were set to 0 when S4 was undetected for a heartbeat. S1 frequency was the most specific feature for E/e′. QS1 had the highest sensitivity (0.83) and AUC (0.72) among all features. N-terminal pro-brain natriuretic peptide showed a similar diagnostic performance to any eligible PCG features (P > 0.05).
Table 3.
Phonocardiography for prediction of E/e′ > 9 in matched patients (n = 25)
| Cut-off value | Sensitivity | Specificity | AUC | |
|---|---|---|---|---|
| S4 amplitude | 6.6 × 10−4 | 0.83 | 0.62 | 0.69 (0.47–0.86) |
| S1 frequency | 46.9 Hz | 0.50 | 0.92 | 0.68 (0.47–0.85) |
| S2 frequency | 45.9 Hz | 0.67 | 0.54 | 0.56 (0.35–0.76) |
| S4 frequency | 31.3 Hz | 0.83 | 0.69 | 0.69 (0.48–0.86) |
| S4% | 73 | 0.83 | 0.77 | 0.69 (0.48–0.86) |
| S3 and S4% | 53 | 0.83 | 0.62 | 0.70 (0.48–0.86) |
| QS1 | 64 ms | 0.83 | 0.69 | 0.72 (0.51–0.88) |
| QS2c | 415 ms | 0.67 | 0.77 | 0.67 (0.46–0.85) |
| NT-proBNP | 152 pg/mL | 0.83 | 0.54 | 0.67 (0.46–0.85) |
Phonocardiographic features deemed predictive of E/e' > 9 are shown in bold. See the main text for more explanations. AUC, area under the curve; NT-proBNP, N-terminal pro-brain natriuretic peptide; QS1, Q to S1 onset; QS2c, Q to S2 onset corrected for RR interval; S1, first heart sound; S2, second heart sound; S4%, percentage of heartbeats with S4.
Discussion
The main findings of this pilot study on the relationship between HS and echocardiography in patients suspected of HFpEF are as follows: (i) higher HS frequencies (S1, S2, and S4), S4 amplitude and occurrence, and a longer QS2c were related to elevation of LV filling pressure indicated by E/e′ and (ii) QS1 was the best marker for E/e′ > 9. Our findings provide clues for using HS to assess HFpEF patients.
Heart sound as a novel marker of heart failure with preserved ejection fraction
Our findings support HS as a novel simple marker of E/e′ elevation in patients suspected of HFpEF. The higher S1 and S2 frequencies in the patients with an elevated E/e′ ratio are consistent with the idea that stiffened ventricles are linked to higher HS frequencies.18 Further evidence came from the more frequent occurrence of S4 which has been well established as a marker of less compliant ventricles.19–21
The reasons for the stiffened ventricles are multifaceted. Firstly, the structural cause may come from myocardial hypertrophy. In our matched patients, the LV mass and mass index were significantly higher in the patients with a high E/e′ ratio. Secondly, the higher stiffness may relate to an increased ventricular wall tension, as suggested by a larger end-diastolic diameter and left atrial volume in the patients with a high E/e′. A high E/e′ per se also indicates an increased LV filling pressure.22 This relationship has been supported by a recent open-chest porcine study in which ventricular vibrations were collected using an epicardially attached accelerometer.23 The study demonstrated a close relationship between changes in S1 frequency and changes in end-diastolic volume during fluid administration to alter ventricular preload and myocardial tension. Compared with hypertrophy, wall tension is likely reversed as the patients’ conditions are improved with treatments, indicating the value of HS frequency in monitoring the patients’ disease conditions, yet this idea awaits further investigation.
Causes of longer QS1 and QS2 in E/e′ elevation
Another finding of the present study was that HFpEF patients with a higher E/e′ also tended to have longer QS1 and QS2 intervals. QS1 reflects the time required for the ventricles to build up sufficient intracardiac pressure to close the atrioventricular valves. Thus, prolongation of QS1 is associated with slower myocardial force development and/or elevated atrial pressure. Weakened myocardial force does not seem to be a major contributor of a longer QS1 in the high E/e′ group because this group had a higher LVEF compared with the low E/e′ group (62 vs. 58%). Therefore, the prolonged QS1 interval in patients with high E/e′ is most likely related to an increased left atrial pressure. The elevated left atrial pressure not only increases the trans-atrioventricular valve pressure gradient but also widely separates the atrioventricular valves at the moment of ventricular electrical activation. Prolongation of QS1 may also come from a shortened PR interval, but this possibility seems unlikely because the matched groups had similar PR intervals.24 Therefore, QS1 may be a marker tailored for differentiating HFpEF patients who are characterized by an increased LV filling pressure rather than a weakened myocardial force. This hypothesis is confirmed by the highest diagnostic performance of QS1 among all PCG features and NT-proBNP for prediction of E/e′ over 9.
A longer QS2 in the high E/e′ group indicates a delayed total electromechanical systolic time. QS2 lengthening appears to be the result of both prolonged QS1 and S1S2 intervals. While the prolongation of QS1 has been discussed previously, the prolongation of S1S2 is likely caused by longer ejection time, supported by the higher LVEF in the high E/e′ group. Another cause of a longer QS2 and QS2c may be hypertrophy which has been shown to prolong QT interval on ECG.25 However, this relationship needs to be further investigated.
Advantages of heart sound for heart failure with preserved ejection fraction
Our findings show that PCG features are non-inferior to NT-proBNP in differentiating E/e′ ratios. In the matched patients, S4% and QS1 showed the same sensitivity as NT-proBNP. However, HS is advantageous in its non-invasiveness compared with serum NT-proBNP measurement. Secondly, HS measurement is time-efficient. In our study, an HS recording took 15–30 s. This short time enables a simple evaluation of E/e′ elevation in an acute setting feasible. Thirdly, HS measurement is cost-effective. The device used for HS collection such as the EKO digital stethoscope in the current study costs only a few hundreds of dollars and can be used as long as the device is charged. In comparison, NT-proBNP measurement requires buying the detection kit, while an echocardiography machine is generally sold at a much higher price than a digital stethoscope. Finally, the EKO device can be used by patients at home to record ECG and HS which may be remotely interpreted by the cardiologist/nurse, reducing hospital visits and thus healthcare costs. The above advantages make HS a promising tool for HFpEF evaluation. Hence, future studies focusing on implementing digital stethoscopes for HFpEF diagnosis are warranted.
Current heart sound studies of heart failure with preserved ejection fraction
Overall, studies of HS in HFpEF patients have been clearly overlooked considering the long history of studying HS in patients with ventricular systolic dysfunction. Recent popularity of machine learning has enabled the data-driven studies of HS for the classification of heart failure by LVEF (i.e. normal, reduced, and preserved).9–11,26 Early machine learning studies relied on the manual extraction of HS features for training the algorithm. For example, extreme learning machines trained by HS features such as diastolic-to-systolic duration ratio showed high sensitivity (95%) and specificity (97%) in classification between HFpEF patients and healthy controls.9 Deep learning takes the advantage of automatic feature extraction from raw HS signals for training the model. A gated recurrent unit has been used to automatically learn the deep features from the raw signals and shown an accuracy of 99% in classification among HFpEF, HFrEF, and normal controls.11 Convolutional neural network trained with a short-time Fourier transform spectrum is reported to show 99% of sensitivity and specificity in distinguishing LV diastolic dysfunction (n = 30) from healthy controls (n = 41).10 The LV diastolic dysfunction group was a mixture of HFrEF and HFpEF patients (LVEF: 45% ± 16%) with a high E/e′ ratio (18.6 ± 6.7).
Although machine learning is not investigated in our present study, given its high accuracy reported in other studies, it will conceivably further improve the prediction power of our proposed HS features. Our findings on the relationship between HS and echocardiography provide interpretable PCG features as inputs for training machine learning models. Deep learning takes the advantage of automatic feature extraction and may be another promising approach for the prediction of E/e′ and classification of HFpEF from the normal. In this case, time–frequency representation of HS helps better visualize HS patterns and serves as an input to the deep learning model. However, deep learning requires a larger sample size in future studies.
Limitations
Several limitations need to be addressed in the future. Firstly, this single-centre pilot study had a small sample size which limited the generalizability of our findings. A larger-scale study helps enrol a more representative group of patients, thus allowing constructing an HS score to better classify the patients. It would also allow revealing the relationship between PCG features and patients’ outcomes. Secondly, a cut-off value of 45% rather than 50% was chosen for LVEF in this study to include patients suspected of HFpEF who, in the end, appeared to have heart failure with midrange ejection fraction in a borderline region with HFpEF. In fact, only one patient had an LVEF between 45 and 50% (48%), and the patient was not different from the other patients regarding baseline characteristics, echocardiographic parameters, or PCG features and did not lead to notably different diagnostic performances. Thirdly, the patients included in the current study appeared to have a mild degree of diastolic dysfunction, while HFpEF patients of an advanced stage or during a decompensated state may present an E/e′ over 15.27 However, the fact that HS can differentiate ‘borderline’ E/e′ values may also prove valuable for preliminary assessment of the patients. Moreover, considering E/e′ as a continuous rather than dichotomous variable is likely more valuable to clinical practice but also requires a larger sample size. Lastly, although E/e′ is reported as the best-established echocardiographic diastolic parameter, the correlation between E/e′ and invasive filling pressures may not be perfect in HFpEF patients.28 A proper approach would be to simultaneously measure heart sounds and LV pressure during invasive procedures.
Conclusions
Phonocardiography features such as HS frequency and timing intervals are related to E/e′ in patients suspected of HFpEF. The associations between heart sounds and diastolic dysfunction need to be further clarified in future studies.
Supplementary Material
Acknowledgements
We would like to thank Jaeson Bang, founder of Future Cardia, for providing the EKO digital stethoscopes for this study.
Contributor Information
Hongxing Luo, Department of Physiology, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, Universiteitssingel 50, 6229 ER Maastricht, The Netherlands.
Jerremy Weerts, Department of Cardiology, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University Medical Centre (MUMC+), P. Debyelaan 25, 6229 HX Maastricht, The Netherlands.
Anja Bekkers, Department of Cardiology, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University Medical Centre (MUMC+), P. Debyelaan 25, 6229 HX Maastricht, The Netherlands.
Anouk Achten, Department of Cardiology, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University Medical Centre (MUMC+), P. Debyelaan 25, 6229 HX Maastricht, The Netherlands.
Sien Lievens, Department of Physiology, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, Universiteitssingel 50, 6229 ER Maastricht, The Netherlands; Department of Cardiology, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University Medical Centre (MUMC+), P. Debyelaan 25, 6229 HX Maastricht, The Netherlands.
Kimberly Smeets, Department of Cardiology, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University Medical Centre (MUMC+), P. Debyelaan 25, 6229 HX Maastricht, The Netherlands.
Vanessa van Empel, Department of Cardiology, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University Medical Centre (MUMC+), P. Debyelaan 25, 6229 HX Maastricht, The Netherlands.
Tammo Delhaas, Department of Biomedical Engineering, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, Universiteitssingel 50, 6229 ER Maastricht, The Netherlands.
Frits W Prinzen, Department of Physiology, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, Universiteitssingel 50, 6229 ER Maastricht, The Netherlands.
Supplementary material
Supplementary material is available at European Heart Journal – Digital Health.
Funding
This study was funded by a grant from the European Union Horizon 2020 research and Innovation programme ‘Personalised In-silico Cardiology (PIC)’ under the H2020 Marie Skłodowska-Curie Actions grant agreement no. 764738.
Data availability
All data are included in the submission/manuscript file.
References
- 1. Dunlay SM, Roger VL, Redfield MM. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol 2017;14:591–602. [DOI] [PubMed] [Google Scholar]
- 2. Borlaug BA. The pathophysiology of heart failure with preserved ejection fraction. Nat Rev Cardiol 2014;11:507–515. [DOI] [PubMed] [Google Scholar]
- 3. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. . 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599–3726. [DOI] [PubMed] [Google Scholar]
- 4. Hill SA, Booth RA, Santaguida PL, Don-Wauchope A, Brown JA, Oremus M, et al. . Use of BNP and NT-proBNP for the diagnosis of heart failure in the emergency department: a systematic review of the evidence. Heart Fail Rev 2014;19:421–438. [DOI] [PubMed] [Google Scholar]
- 5. Bachtiger P, Petri CF, Scott FE, Park SR, Kelshiker MA, Sahemey HK, et al. . Point-of-care screening for heart failure with reduced ejection fraction using artificial intelligence during ECG-enabled stethoscope examination in London, UK: a prospective, observational, multicentre study. Lancet Digit Health 2022;4:e117–e125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Inan OT, Pouyan PM, Javaid AQ, Dowling S, Etemadi M, Dorier A, et al. . Novel wearable seismocardiography and machine learning algorithms can assess clinical status of heart failure patients. Circ Heart Fail 2018;11:e4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Drazner MH, Rame JE, Stevenson LW, Dries DL. Prognostic importance of elevated jugular venous pressure and a third heart sound in patients with heart failure. N Engl J Med 2001;345:574–581. [DOI] [PubMed] [Google Scholar]
- 8. Kono T, Rosman H, Alam M, Stein PD, Sabbah HN. Hemodynamic correlates of the third heart sound during the evolution of chronic heart failure. J Am Coll Cardiol 1993;21:419–423. [DOI] [PubMed] [Google Scholar]
- 9. Liu Y, Guo X, Zheng Y. An automatic approach using ELM classifier for HFpEF identification based on heart sound characteristics. J Med Syst 2019;43:285. [DOI] [PubMed] [Google Scholar]
- 10. Yang Y, Guo XM, Wang H, Zheng YN. Deep learning-based heart sound analysis for left ventricular diastolic dysfunction diagnosis. Diagnostics (Basel) 2021;11:2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gao S, Zheng Y, Guo X. Gated recurrent unit-based heart sound analysis for heart failure screening. Biomed Eng Online 2020;19:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Weerts J, Barandiarán Aizpurua A, Henkens MTHM, Lyon A, van Mourik MJW, van Gemert MRAA, et al. . The prognostic impact of mechanical atrial dysfunction and atrial fibrillation in heart failure with preserved ejection fraction. Eur Heart J Cardiovasc Imaging 2021;23:74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. . Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015;16:233–270. [DOI] [PubMed] [Google Scholar]
- 14. Vandenberk B, Vandael E, Robyns T, Vandenberghe J, Garweg C, Foulon V, et al. . Which QT correction formulae to use for QT monitoring? J Am Heart Assoc 2016;5:e003264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Weissler AM, Harris WS, Schoenfeld CD. Systolic time intervals in heart failure in man. Circulation 1968;37:149–159. [DOI] [PubMed] [Google Scholar]
- 16. Chen JW, Maldonado DR, Kowalski BL, Miecznikowski KB, Kyin C, Gornbein JA, et al. . Best practice guidelines for propensity score methods in medical research: consideration on theory, implementation, and reporting. A review. Arthroscopy 2022;38:632–642. [DOI] [PubMed] [Google Scholar]
- 17. Youden WJ. Index for rating diagnostic tests. Cancer 1950;3:32–35. [DOI] [PubMed] [Google Scholar]
- 18. Adolph RJ, Stephens JF, Tanaka K. The clinical value of frequency analysis of the first heart sound in myocardial infarction. Circulation 1970;41:1003–1014. [DOI] [PubMed] [Google Scholar]
- 19. Shah SJ, Nakamura K, Marcus GM, Gerber IL, McKeown BH, Jordan MV, et al. . Association of the fourth heart sound with increased left ventricular end-diastolic stiffness. J Card Fail 2008;14:431–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Voon WC, Sheu SH, Hwang YY. Doppler study of transmitted transmitral a wave in patients with a fourth heart sound. Echocardiography 1997;14:243–259. [DOI] [PubMed] [Google Scholar]
- 21. Van de Werf F, Minten J, Carmeliet P, De Geest H, Kesteloot H. The genesis of the third and fourth heart sounds. A pressure-flow study in dogs. J Clin Invest 1984;73:1400–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mitter SS, Shah SJ, Thomas JD. A test in context: E/A and E/e′ to assess diastolic dysfunction and LV filling pressure. J Am Coll Cardiol 2017;69:1451–1464. [DOI] [PubMed] [Google Scholar]
- 23. Krogh MR, Halvorsen PS, Grymyr OHN, Bergsland J, Elle OJ, Fosse E, et al. . Continuous estimation of acute changes in preload using epicardially attached accelerometers. IEEE Trans Biomed Eng 2021;68:2067–2075. [DOI] [PubMed] [Google Scholar]
- 24. Bashour TT, Naughton JP, Cheng TO. Systolic time intervals in patients with artificial pacemakers. Noninvasive technique for assessing atrial contribution to stroke volume at various P-R intervals. Am J Cardiol 1973;32:287–290. [DOI] [PubMed] [Google Scholar]
- 25. Dritsas A, Sbarouni E, Gilligan D, Nihoyannopoulos P, Oakley CM. QT-interval abnormalities in hypertrophic cardiomyopathy. Clin Cardiol 1992;15:739–742. [DOI] [PubMed] [Google Scholar]
- 26. Zheng Y, Guo X, Qin J, Xiao S. Computer-assisted diagnosis for chronic heart failure by the analysis of their cardiac reserve and heart sound characteristics. Comput Methods Programs Biomed 2015;122:372–383. [DOI] [PubMed] [Google Scholar]
- 27. Pieske B, Tschöpe C, de Boer RA, Fraser AG, Anker SD, Donal E, et al. . How to diagnose heart failure with preserved ejection fraction: the HFA-PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur Heart J 2019;40:3297–3317. [DOI] [PubMed] [Google Scholar]
- 28. Nauta JF, Hummel YM, van der Meer P, Lam CSP, Voors AA, van Melle JP. Correlation with invasive left ventricular filling pressures and prognostic relevance of the echocardiographic diastolic parameters used in the 2016 ESC heart failure guidelines and in the 2016 ASE/EACVI recommendations: a systematic review in patients with heart failure with preserved ejection fraction. Eur J Heart Fail 2018;20:1303–1311. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are included in the submission/manuscript file.