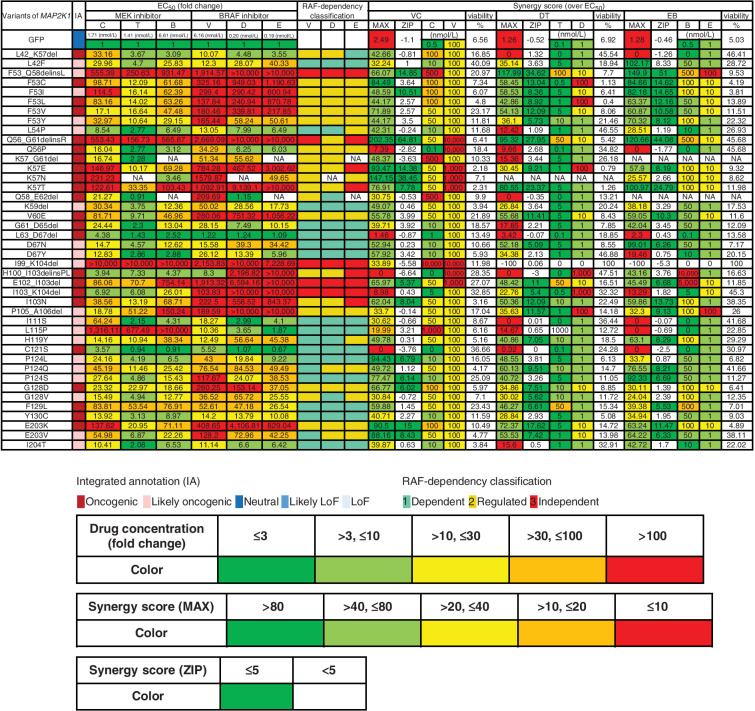
Figure 5.
Assessment of drug sensitivity and synergy for oncogenic MAP2K1 variants. The drug sensitivities of the indicated oncogenic MAP2K1 variant mutants were categorized into five levels; ≤ 3 (green); > 3, ≤ 10 (yellow-green); > 10, ≤ 30 (yellow); > 30, ≤ 100 (orange); and > 100 (red) based on the fold change (to GFP) of the IC50 value for each drug against each mutant. The maximum synergistic effect among the combinations of concentrations above the IC50 was calculated and concentrations of BRAF and MEK inhibitors in the case shown on the right side were categorized into five levels; > 80 (green); > 40, ≤ 80 (yellow-green); > 20, ≤ 40 (yellow); > 10, ≤ 20 (orange); and ≤ 10 (red). The concentration of the drug in each case is displayed (nmol/L) and colored on the basis of the fold change of IC50 for GFP. The mean of the synergy effect is shown in the ZIP, where ≥ 5 (green) is defined as having a synergy effect. B, binimetinib; C, cobimetinib; D, dabrafenib; DT, dabrafenib and trametinib; E, encorafenib; EB, encorafenib and binimetinib; EC50, half maximal effective concentration; NA, not applicable; T, trametinib; V, vemurafenib, VC, vemurafenib and cobimetinib.