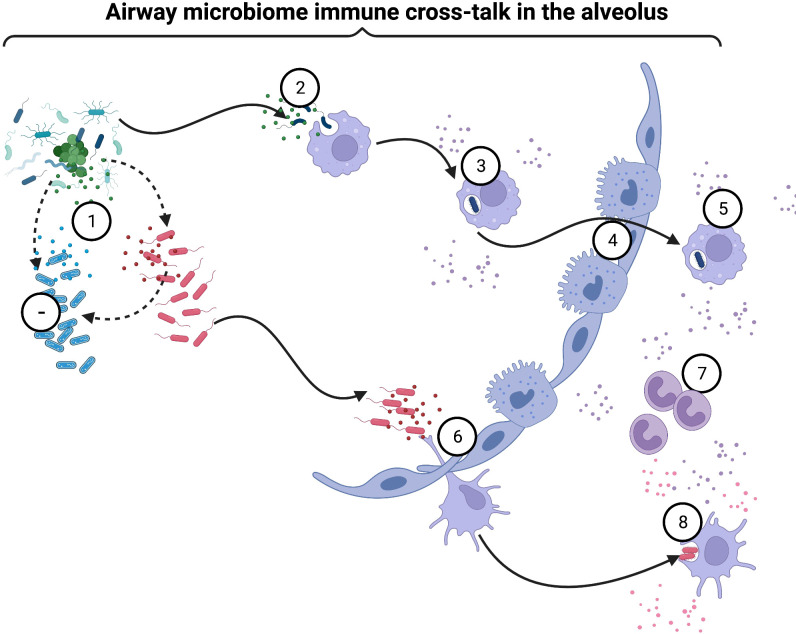
Figure 3.
Airway microbiome immune crosstalk in alveoli: Following successful colonization of the respiratory tract, bacterial species replicate rapidly and establish dense biological communities (1). Some bacteria inhibit other species via the secretion of antimicrobial peptides and lytic enzymes(1). Alveolar macrophages engulf bacteria(2) and become activated via MyD88-dependent and -independent mechanisms(3). Airway microbiome sensing potentiates bacterial killing(3). Some intracellular pathobionts infect alveolar macrophages and access the lung interstitium (4). Activated macrophages secrete chemoattractants and promote the migration of other cellular players, such as monocytes, neutrophils, and adaptive immune response cells, into the airways to clear pathobionts (5). Dendritic cells (DCs) continuously sample airway bacteria that attach and colonize the mucosa via protruding dendrites(6). Airway microbiome sensing by bronchial epithelial cells, alveolar macrophages, and DCs activate innate lymphoid cells (ILCs) (7), which modulate other immune cells’ activity. Depending on the type and degree of microbial exposure, DCs induce a wide range of immune responses, from immune tolerance induced by plasmacytoid DCs (pDCs) to inflammation induced by conventional DCs (cDCs)(8). Continuous microbial sampling and trafficking by activated DCs, and alveolar macrophages deliver processed microbial antigens to naïve CD4+T and CD8+T cells within mucosa-associated lymphoid tissue (MALT) and draining lymph nodes (8). Created with BioRender.com.