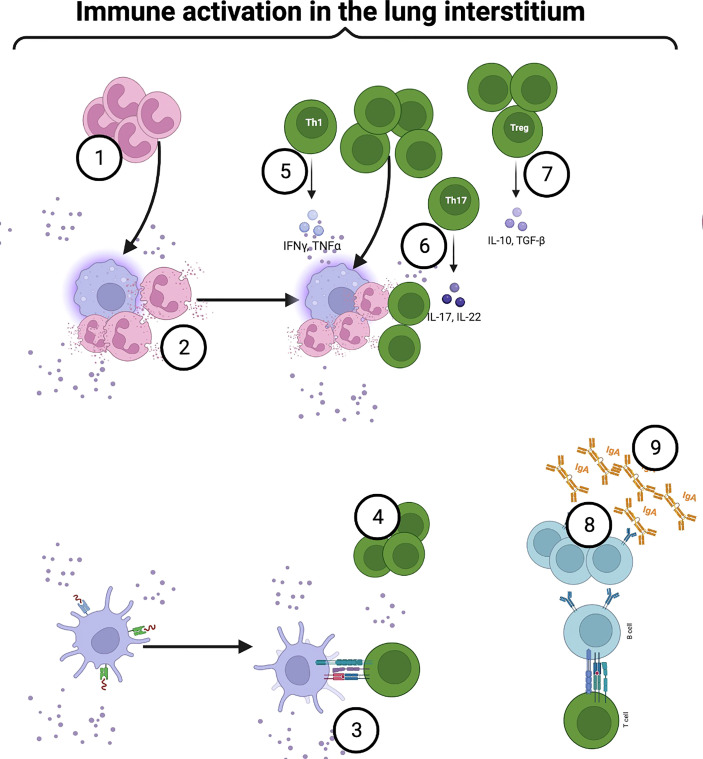
Figure 4.
Airway microbiome immune crosstalk in the lung interstitium: Some intracellular pathobionts infect alveolar macrophages and access the interstitium. Activated macrophages secrete chemoattractants and promote neutrophilic infiltration into the airways to clear pathobionts (1). Upon bacterial sensing, neutrophils become activated, degranulate and release NETs (2). Continuous sampling and trafficking by activated DCs, and alveolar macrophages deliver processed microbial antigens to naïve CD4+T within mucosa-associated lymphoid tissue (MALT) and draining lymph nodes (4). Under a steady state, mature lung cDCs are preferentially programmed to induce a Th2 immune response. However, following immune sensing and activation, the production of IL-12, IL-23, IL-27, and notch ligand by airway DCs, alveolar macrophages, and epithelial cells induce a Th1 response (5). Among CD4+T cell phenotypes, microbiome-mediated mucosal inflammation has been strongly linked to aberrant Th17 (6). Besides the Th17 phenotype, microbial interaction with mucosal CD4+T cells induces immune tolerance (7). MyD88-dependent TLR2 activation by capsular polysaccharide A induces the expansion of Foxp3+T cells within the mucosa (7). Foxp3+T cells drive IL-10 production, facilitating mucosal immune tolerance (7). In another mechanism, microbial-induced Tregs promote mucosal memory B or plasma cells’ IgA secretion (8). Created with BioRender.com.