Figure 8. Enhanced formation of ataxin‐1 nuclear inclusions in stress granule‐deficient cells.
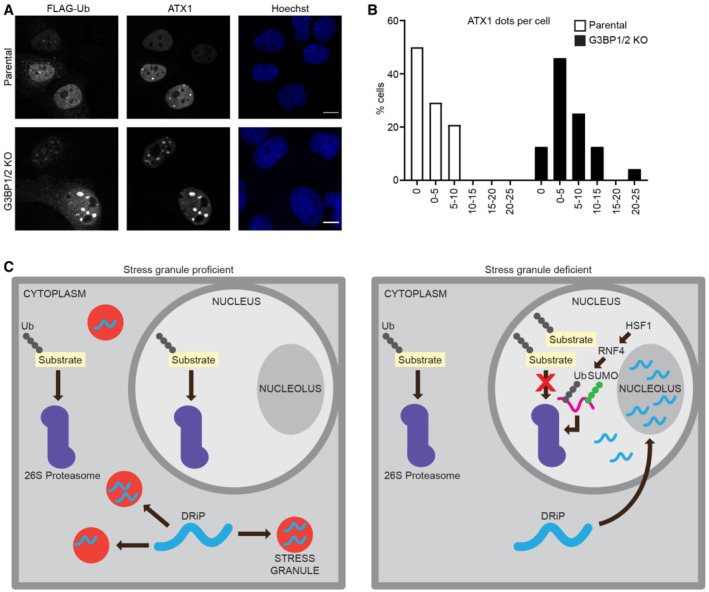
- Representative confocal images of immunofluorescent staining of FLAG‐tagged ubiquitin (FLAGUb) and mutant ataxin1 (ATX1) in parental and G3BP1/2 knockout U2OS cells. Cells were subjected to a heat shock followed by 4 h recovery. Scale bar, 10 μm.
- Frequency distribution of ataxin‐1 inclusions per cell from three independent experiments.
- Schematic drawing of the model. In the absence of stress granules, proteotoxic stress causes DRiPs to translocate from the cytosol to the nucleus where they accumulate in nucleoli and sequester Hsp70. This results in early activation of HSF1 and RNF4 and targeting of SUMOylated proteins for proteasomal degradation. These substrates overload the nuclear UPS impairing the degradation of other ubiquitin‐dependent proteasome substrates.
