Abstract
Successful sentence comprehension requires the binding, or composition, of multiple words into larger structures to establish meaning. Using magnetoencephalography, we investigated the neural mechanisms involved in binding at the syntax level, in a task where contributions from semantics were minimized. Participants were auditorily presented with minimal sentences that required binding (pronoun and pseudo-verb with the corresponding morphological inflection; “she grushes”) and pseudo-verb wordlists that did not require binding (“cugged grushes”). Relative to no binding, we found that syntactic binding was associated with a modulation in alpha band (8–12 Hz) activity in left-lateralized language regions. First, we observed a significantly smaller increase in alpha power around the presentation of the target word (“grushes”) that required binding (−0.05 to 0.1 s), which we suggest reflects an expectation of binding to occur. Second, during binding of the target word (0.15–0.25 s), we observed significantly decreased alpha phase-locking between the left inferior frontal gyrus and the left middle/inferior temporal cortex, which we suggest reflects alpha-driven cortical disinhibition serving to strengthen communication within the syntax composition neural network. Altogether, our findings highlight the critical role of rapid spatial–temporal alpha band activity in controlling the allocation, transfer, and coordination of the brain’s resources during syntax composition.
Keywords: alpha oscillations, language, connectivity, sentence comprehension, syntax
Introduction
The expressive power of human language is largely derived from our ability to combine multiple words into larger syntactic structures with more complex meaning. Such binding, or compositional, processes occur during the comprehension of even the most basic 2-word phrases (e.g. “she walks”). Characterizing the neural processes involved in composition—also referred to as “Unification” (Hagoort 2003) or “Merge” (Chomsky 1995)—has been a central topic of research for many years (Hagoort 2019; Pylkkänen 2019; Matchin and Hickok 2020). In the present study, we provide novel insight into the neural mechanisms involved in syntax composition using a task in which syntactic binding is dissociable from semantic composition. We use the term “syntactic binding” to specifically refer to the neural processes involved in the combining of individual words into larger structures. We employed a minimal phrase paradigm involving pseudo-words (i.e. following the phonotactic rules of a language, but not conveying semantic meaning).
Sentential compositional processes predominantly occur within a left-lateralized network of brain regions, including the inferior frontal gyrus (IFG) and angular gyrus (Friederici et al. 2000; Humphries et al. 2006; Pallier et al. 2011; Matchin et al. 2017). Within this network, modulations in theta, alpha, and beta frequencies are thought to be crucial for higher order linguistic functions (Bastiaansen et al. 2010; Meyer 2018; Prystauka and Lewis 2019). However, the precise neural mechanisms of syntax composition, relating to frequency modulations, remain elusive. This is because while some studies have found compositional processing to be associated with increased alpha and beta power (Meyer et al. 2013; Segaert et al. 2018), others have found sentence unification to be associated with a power decrease (Wang et al. 2012; Lam et al. 2016; Gastaldon et al. 2020). Moreover, it is unclear how functional connectivity (i.e. phase-locked) between the neural oscillations in different brain regions may contribute toward successful syntax composition. Current evidence suggests that functional connectivity between regions implicated in compositional processes, such as the left IFG, anterior temporal lobe (ATL), and posterior superior temporal gyrus, is beneficial for sentence comprehension (Schoffelen et al. 2017; Vassileiou et al. 2018; Lopopolo et al. 2021). However, the studies discussed thus far have typically used complex sentence structures—this means that other cognitive processes, such as working memory, are also involved in comprehending the sentence stimuli (Pylkkänen 2019), thereby making it difficult to functionally isolate the neural processes involved in sentence composition alone. In this study, therefore, we aim to characterize the neural mechanisms of syntax composition, both in terms of power modulations and phase-locked connectivity, at its most basic 2-word level.
A short 2-word sentence (e.g. “red boat”) is a traceable linguistic unit which can be used to decompose the brain networks implicated in the processing of lengthier sentences (Pylkkänen 2019). Research involving minimal sentences has identified a network of left-lateralized brain areas that underlie composition, most notably including the ATL (Bemis and Pylkkänen, 2011, 2013; Pylkkänen et al. 2014; Westerlund et al. 2015; Zhang and Pylkkänen 2015, 2018; Ziegler and Pylkkänen 2016; Schell et al. 2017; Zaccarella et al. 2017; Blanco-Elorrieta et al. 2018; Fyshe et al. 2019; but cf. Kochari et al. 2021). Importantly, in order to more precisely identify the neural processes involved in syntactic binding that are dissociable from semantics, one further approach is to use pseudo-words within a minimal phrase paradigm. Using fMRI, Zaccarella and Friederici (2015) found increased hemodynamic responses in the anterior part of the left pars opercularis (part of the IFG) during comprehension of determiner-noun phrases involving pseudo-nouns (“this flirk”) compared with wordlists involving 1 pseudo-noun (“apple flirk”). Building on this, using EEG, Segaert et al. (2018) found increased alpha and beta power (centralized over frontal-central electrodes) during comprehension of minimal phrases involving pseudo-verbs (“she grushes”) compared with wordlists of 2 pseudo-verbs (“cugged grushes”), which they interpreted as reflecting syntactic binding (see also Poulisse et al. 2020). We aim to build on these findings and use magnetoencephalography (MEG) to precisely characterize and localize the rapid temporal features and functional connectivity within a spatially distributed network of brain regions that support syntax composition independent of semantics.
We focused on frequency ranges up to 30 Hz as it is modulations in these frequencies that are considered critical for syntax composition (e.g. Bastiaansen et al. 2010; Segaert et al. 2018; Gastaldon et al. 2020; Poulisse et al. 2020), whereas semantic composition appears to involve higher gamma band activity (Hald et al. 2006; Bastiaansen and Hagoort 2015). In particular, we hypothesize to observe effects in the alpha (~8–12 Hz) frequency band given previous evidence that modulations in the alpha band reflect sentence compositional processing; however, the directionality of this effect is difficult to predict given that some research has found compositional processing to be associated with increased alpha (Meyer et al. 2013; Segaert et al. 2018), whereas others have found it to be associated with alpha decreases (Wang et al. 2012; Lam et al. 2016; Gastaldon et al. 2020). We may also reasonably expect to observe effects of syntax composition in the theta (~4–7 Hz) and beta (~15–30 Hz) frequency bands if they also contribute toward sentence comprehension (Meyer 2018; Prystauka and Lewis 2019). In line with previous work, we predominantly expect to observe oscillatory effects of syntactic binding immediately preceding and following the onset of the target word where binding occurs (i.e. “grushes”) (Bemis and Pylkkänen 2013; Segaert et al. 2018; Zhang and Pylkkänen 2018; Poulisse et al. 2020). We hypothesize that our observed oscillatory effects will be localized to left hemisphere language regions, including the LIFG (e.g. Hagoort 2003; Tyler and Marslen-Wilson 2008; Zaccarella and Friederici 2015; Zaccarella et al. 2017). However, the more interesting hypotheses relate to the functional connectivity between different language-relevant regions during syntactic binding. In particular, alpha band desynchronization has been found to predict successful sentence encoding (Magazzini et al. 2016; Vassileiou et al. 2018; Alavash et al. 2021), suggesting that alpha desynchronization serves to strengthen communication within a cortical network by enabling the increase transfer of information (Jensen and Mazaheri 2010; Klimesch 2012; Van Diepen et al. 2019). Our study is the first to closely examine functional connectivity among brain regions involved in minimal syntactic binding, but building on previous work, we may predict to observe less alpha-locking during syntactic binding compared with no binding.
Materials and methods
The methods and planned analyses of this study were preregistered on the Open Science Framework prior to data collection (https://osf.io/ntszu).
Participants
We recruited 25 healthy participants: all were right-handed and native monolingual British–English speakers. One participant was excluded due to excessive movement artifacts during the MEG test session (>50% trials removed), meaning that a sample of 24 participants was used in the time–frequency analyses (13 female/11 male, M = 24.2 years, SD = 4.1 years). Anatomical T1 brain scans were acquired for 21 of the participants (2 participants did not attend the MRI session, and another did not complete the MRI session due to unexpected discomfort). Further technical issues with MRI-MEG co-registration with 2 participants meant that a sample of 19 participants was used for the source localization and connectivity analyses (10 females/9 males, M = 24.1 years, SD = 4.2 years). The study was approved by the University of Birmingham Ethical Review Committee. All participants provided written informed consent and were compensated monetarily.
Experimental design and stimuli
We employed a simple design of 2 experimental conditions: the sentence condition consisting of a minimal 2-word phrase (pronoun plus pseudo-verb) for which it was highly likely that syntactic binding may plausibly occur (e.g. “she grushes”); and the wordlist condition consisting of 2 pseudo-verbs for which syntactic binding was highly unlikely to occur (e.g. “cugged grushes”). Syntactic binding occurred in the sentence condition (but not the wordlist condition) because the correct morphological inflection (i.e. −es) cued binding with the corresponding pronoun, in a way that is similar to how an intransitive verb phrase may be interpreted (e.g. “she grushes” is akin to “she walks”). Varying the first word between the sentence and wordlists conditions (while ensuring that the second word was matched across the 2 conditions) enabled us to experimentally manipulate the binding context of the second word (syntactic binding vs. no binding). Our analyses therefore specifically focus on the neural signature surrounding the presentation of the second word only as this is the time period of interest where we expect binding to occur, matching the approach taken in other minimal binding studies (Bemis and Pylkkänen 2011, 2013; Zaccarella and Friederici 2015; Schell et al. 2017; Segaert et al. 2018; Zhang and Pylkkänen 2018). Furthermore, we followed the approach of the MEG/EEG binding literature of not analyzing the first word when it is not possible to match it between conditions (e.g. Bemis and Pylkkänen 2011; Segaert et al. 2018).
Behavioral evidence from previous use of this paradigm has shown that participants judge pseudo-verb sentences with the correct morphological inflection to be valid sentences (e.g. “she grushes”) but judge sentences with the incorrect inflection (e.g. “she grush,” “I grushes”) and pseudo-verb wordlists (e.g. “cugged grushes”) to be invalid sentences (Poulisse et al. 2019, 2020). This is evidence that listeners do not attempt to bind the 2 pseudo-verbs together when in a wordlist as, if they did, they would judge it to be a valid sentence (e.g. if they had interpreted “cugged grushes” as a deverbal adjective and noun pairing). We therefore consider it highly likely that participants in our study were engaging in syntactic binding when a minimal sentence was presented, but not when a wordlist was presented (in line with the assumptions of other studies of minimal binding; Bemis and Pylkkänen 2011, 2013; Segaert et al. 2018; Blanco-Elorrieta et al. 2018; Poulisse et al. 2020). Moreover, following the neuroimaging binding literature, we did not include a behavioral judgment task within our MEG study because it is very important that there is no difference in judgment between the conditions for which the EEG/MEG signal is compared, otherwise judgment (yes/no) and condition (binding/no binding) would be conflated, making it impossible to directly compare the signatures of the 2 conditions.
To construct the experimental items, we used a set of 20 pseudo-verbs created by Ullman et al. (1997): brop, crog, cug, dotch, grush, plag, plam, pob, prap, prass, satch, scash, scur, slub, spuff, stoff, trab, traff, tunch, vask (root forms, un-inflected). All pseudo-verbs were monosyllabic and could be inflected according to the grammatical rules of regular English verbs. We combined each pseudo-verb with 3 different morphological affixes (no affix; +s; +ed) to create 60 possible pseudo-verb-affix combinations. In English, only certain pronouns may be combined with certain affixes (e.g. “she grushes” is acceptable, but “I grushes” is not). Using a list of 6 pronouns (I, you, he, she, they, we), we created 120 sentence items by pairing each pseudo-verb-affix with 2 different pronouns that were syntactically appropriate for the corresponding affix, such that syntactic binding may plausibly occur (e.g. “I dotch,” “she grushes,” “they cugged”). To create the wordlist items, we paired together 2 different pseudo-verb-affix stimuli for which no syntactic binding occurred (e.g. “cugged grushes,” “dotch traffed”). Each pseudo-verb-affix stimulus occurred twice as the first word in a pair and twice as the second word in a pair, creating a total of 120 wordlist items. We ensured that the 2 words within each wordlist pair always consisted of a different pseudo-verb and a different affix.
We also created 120 filler items. Sixty of the fillers consisted of reversed speech and were included as a detection task for the participants. We reversed the speech of each of the 60 pseudo-verb-affix combinations and then paired each with either a nonreversed pseudo-verb-affix or pronoun (half as the first word of the pair and half as the second word). A further 60 filler items were used to increase the variability of stimuli presented to participants (Roland et al. 2007). Thirty such items consisted of 2 pronouns (e.g. “she I”); this contrasted the experimental sentence items in which a pronoun was followed by a pseudo-verb (e.g. “she grushes”). The other 30 items consisted of a pseudo-verb-affix stimulus followed by 1 of 5 possible adverbs (early, promptly, quickly, rarely, safely; e.g. “cugged quickly”); this contrasted the experimental wordlist items in which a pseudo-verb was followed by another pseudo-verb (e.g. “cugged grushes”). Overall, this set of fillers created a task and variability for the participants—the filler items did not act as a control to the experimental items and were not analyzed.
All auditory stimuli were spoken by a native English male speaker and normalized to 1-db volume. Each experimental item consisted of separate audio files for word 1 and word 2. Within each audio file, the onset of the word began at exactly 0 s—we achieved this using the audio-editing software Praat (Boersma 2001).
Experimental procedure
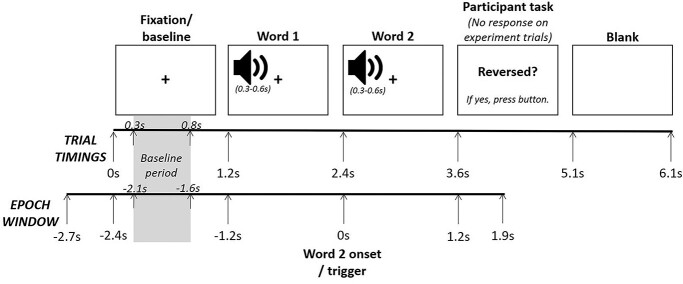
The participants’ task was to detect the reversed speech (which only occurred on filler trials). On each trial, participants were auditorily presented with a 2-word phrase (Fig. 1). At the beginning of each trial, a fixation cross was presented on screen for 1.2 s with no audio—this represented our baseline period. At 1.2 s, the onset of the first word auditory presentation started. The length of word 1 audio varied between 0.3 and 0.6 s. There was then a brief pause before the onset of word 2 audio presentation began, exactly 1.2 s after the word 1 onset (i.e. at 2.4 s in the overall trial timings). This time interval means that the lexical processing of word 1 (which occurs within the first 0.6 s of auditory onset; MacGregor et al. 2012) will have been completed before the onset of word 2. Again, the length of the word 2 audio varied between 0.3 and 0.6 s. The fixation crossed remained on screen throughout the word 1 and word 2 presentation to limit participants’ eye movements. Following the word 2 audio presentation, there was a brief pause until at exactly 1.2 s after the word 2 onset (i.e. at 3.6 s in the overall trial timings), the phrase “Reversed?” appeared on the screen—this was the participant’s decision task. Participants were instructed to press a button if part of the speech was reversed (half of the participants used their left index finger, and half used their right index finger), but to do nothing if the speech was not reversed. There was no difference in response decision processes (i.e. no button press) between the critical experimental conditions of interest (sentence vs. wordlist). The “Reversed?” question remained on screen for 1.5 s (in which time the participant pressed a button or did nothing). Following this, the screen went blank for 1 s before the next trial began with the presentation of the fixation cross. In total, each trial lasted 6.1 s. Each participant completed 360 trials (consisting of 240 experimental trials and 120 filler trials) in a unique randomized order, divided into 6 blocks of 60 trials each. Before beginning the task, participants completed 23 practice trials that were similar to the experimental and filler items used in the main task.
Fig. 1.
Stimuli presentation timings per trial and the related epoch window. The trial timing shown in the figure relates to the onset of the stimuli. The length of the audio files of word 1 and word 2 varied between 0.3 and 0.6 s. Stimuli presentation and trigger signals were controlled using E-prime (Schneider et al. 2002). Visual stimuli were presented using a PROPixx projector, and auditory stimuli were presented using the Elekta audio system and MEG-compatible ear phones. Participants’ motor responses were recorded using an NAtA button pad.
As expected, participants were highly accurate at detecting the reverse speech on the filler trials (M = 94.6%, SD = 2.3%, Range = 82–98%), indicating that they were closely listening to the filler and experimental stimuli throughout (since they did not know when the reversed speech would be presented).
Data acquisition
During the task, ongoing MEG data were recorded using the TRIUX™ system from Elekta (Elekta AB, Stockholm, Sweden). This system has 102 magnetometers and 204 planar gradiometers. These are placed at 306 locations, each having 1 magnetometer and a set of 2 orthogonal gradiometers. The data were collected using a sampling rate of 1,000 Hz and were stored for offline analyses. Prior to sampling, a low-pass filter of ~250 Hz was applied. Four head position indicator coils (HPIs) were placed behind the left and right ear, as well as on the left and right forehead just below the hairline. The positions of the HPIs, the nasion, the left and right preauricular points, as well as the surface points of the scalp, were digitized using a Polhemus™ 3D device to facilitate later coregistration with anatomical brain scans. Additional electrooculography (EOG) and electrocardiogram (ECG) data were collected using methods compatible with the TRIUX™ system.
Anatomical high-resolution T1 brain images were acquired for participants at a later session using a MAGNETOM Prisma 3T MRI system from Siemens (Siemens Healthcare, Erlangen, Germany). These images were used for the reconstruction of individual head shapes to create forward models for the source localization analyses.
MEG preprocessing
The offline processing and analyses of the data were performed using functions from the Fieldtrip software package (Oostenveld et al. 2011) and custom scripts in the MATLAB environment. First, we applied a 0.1-Hz high-pass filter to the MEG data to remove slow-frequency drift in the data. The data were segmented into epochs aligned to the onset of the auditory presentation of the second word from −2.7 to 1.9 s (see Fig. 1) and demeaned. We applied a baseline-subtraction in the time domain to the data (i.e. each trial was subtracted by the mean activity −0.1 to 0 s prior to the onset of the fixation). We ran automatic artifact rejection routine implemented in Fieldtrip to remove superconducting quantum interference device (SQUID) jumps.
We first removed all filler trials as we were specifically interested in the difference in neural responses between the experimental sentence and wordlist conditions (i.e. our contrast of interest). We further removed experimental trials for which the participant incorrectly responded with a button press (i.e. indicated that the speech was reversed when it was not) and experimental trials during which the participant made an accidental button press before the response screen (i.e. during the fixation cross and/or auditory presentation). We then visually inspected the waveforms of each experimental trial and removed trials that contained excessive signal artifacts (e.g. large sensor jumps or gross motor movement by the participant); on average, we removed 16% of experimental trials (38.3/240) per participant (Range = 4–47%). Following all this, there was an average of 101 sentence trials (SD = 12.2) and 100 wordlist trials (SD = 11.6) per participant that were usable for the analyses (out of a maximal 120 trials per experimental condition).
We further removed any persistently poor channels that contained excessive noise or flatlined (Mean channels removed per participant = 2.46; SD = 2.86; Range = 0–9). We then used a spline interpolation weighted neighborhood estimate to interpolate across the removed channels per participant. Ocular and cardiac artifacts were removed from the data using an independent component analysis (Mean artifacts removed per participant = 1.75; SD = 0.68; Range = 1–3). We identified these components from their stereotypical topography and time course, as well as by comparisons with the recorded ECG and EOG time courses.
Statistical analyses
Time–frequency
For the frequency range 1–30 Hz (1-Hz steps), we obtained time–frequency representations (TFRs) of power for each trial using sliding Hanning tapers with an adaptive time window of three cycles for each frequency (ΔT = 3/f). This approach has also been used in a number of previous studies (e.g. Whitmarsh et al. 2011; Van Diepen and Mazaheri 2017; Segaert et al. 2018). For each participant, the data for the planar gradiometer pairs was added to create a 102-channel combined planar map in sensor space, and we baseline-corrected the data using the oscillatory activity during the fixation cross presented at the beginning of the trial. Specifically, using the “absolute” baseline parameter in Fieldtrip, we subtracted the mean of the power of each frequency in a 0.5 s period of the fixation cross presentation (that being −2.1 s to −1.6 s in the epoch window) from all other power values. We calculated the TFRs separately per experimental condition for each participant and then averaged across all participants.
We assessed the statistical differences in time-frequency power between the sentence and wordlist conditions across participants using a cluster-level randomization test (incorporated in the Fieldtrip software), which circumvents the type-1 error rate in a situation involving multiple comparisons (i.e. multiple channels and time-frequency points; Maris and Oostenveld 2007). This approach first clusters the data in sensor space depending on whether the contrast between the 2 conditions exceeds a dependent samples t-test threshold of P < 0.05 (two-tailed). In line with Segaert et al. (2018), we used the following pre-defined frequency bands: theta (4–7 Hz), alpha (8–12 Hz), low beta (15–20 Hz) and high beta (25–30 Hz). We considered a cluster to consist of at least 2 significant adjacent combined planar gradiometers. A Monte Carlo p-value of a cluster was then obtained by calculating the number of times the t-statistics in the shuffled distribution is higher than the original t-statistic obtained when contrasting conditions across 1000 random permutations. We first performed the analyses within the time window of interest, centred around the presentation of the second word (−0.5 s to 1 s of the epoch), as this is where we expect to observe time-frequency effects of syntax composition. We then performed the analyses across the complete timeframe of the auditory presentation (−1.3 s to 1.2 s of the epoch).
To ensure that the observed alpha power changes were not just the spectral representation of the event-related fields (ERFs), the ERF components were subtracted from the TFR (Mazaheri and Picton 2005). The subtraction was achieved by first generating the time frequency decomposition of the ERF data for each condition and participant separately. Next, the time frequency power spectra of the ERF were subtracted from the time frequency power spectra of the MEG signal (not baseline corrected) for each condition. The subsequent power changes in the time-frequency domain were used to generate time frequency power spectra differences between experimental conditions (sentence vs. wordlist). We then reanalyzed the ERF-adjusted TRFs using the same statistical methods outlined above.
Source localization
A realistically shaped description of each participant’s brain was constructed using individual head models obtained using the Polhemus 3D digitizer and the acquired MRI anatomical brain scan (where available). Specifically, using the Iterative Closest Point (ICP) algorithm (Besl and McKay 1992) implemented in Fieldtrip, we manually aligned the MRI images to the digitized scalp surface of each individual. The alignment according to the MEG sensor array was done relative to four digitized HPI coils. The MRI images were then segmented in Fieldtrip and a realistically shaped single-shell description of the brain-skull interface was constructed (Nolte 2003).
Source estimation of the time-locked MEG data was performed using a frequency-domain beam-forming approach (dynamic imaging of coherent sources [DICS]; Gross et al. 2001), which uses adaptive spatial filters to localize power in the entire brain. Specifically, we obtained the cross-spectra density (CSD) matrices in each condition; the CSDs were calculated using Fourier transform in combination with multi-tapers (+/− 3 Hz smoothing). Based on our sensor results, we focused our analysis on 10 Hz (7–13 Hz) activity centred around −0.15 to 0.15 s surrounding the onset of the second word. (To preview the time-frequency findings, we found significant effects in the alpha [8–12 Hz] frequency range in the time period surrounding the onset of the second word [−0.05 s to 0.1 s].) We expanded the time window slightly to −0.15 s to 0.15 s as, for the DICS approach, the window needs to be long enough to allow for sufficient frequency activity within the alpha range (recommended at least three cycles; 3/10 Hz = 0.3 s). Our regularization parameter was set to 5%.
The brain volume of each individual participant was discretized to a grid with a 0.8 cm resolution and the lead field was calculated for each grid point. A common filter was calculated for the sentence and wordlist condition and then applied for the data separately for the individual conditions (Whitmarsh et al. 2011; Mazaheri et al. 2014). The source estimates of the individual participants’ functional data along with the individual anatomical MRI images were warped into a Montreal Neurological Institute (MNI) standard brain (Quebec, Canada; http://www.bic.mni.mcgill.ca/brainweb) before averaging and statistics.
We performed cluster-based randomization tests to identify the grid-points in which there was a significant difference in power between the 2 experimental conditions, guided by the significant findings of the time-frequency analyses (i.e. alpha activity surrounding the onset of the second word) as is a common approach within the field (Wang et al. 2012, 2017; Magazzini et al. 2016). We followed the approach taken in previous MEG literature by directly comparing the 2 conditions without a baseline correction (Mazaheri et al. 2009; Magazzini et al. 2016; Wang et al. 2017). We used these clusters to identify specific regions of interest (ROIs) of the condition difference. We derived anatomical labels of these regions from Brodmann’s map and from the Automated Anatomical Labelling Atlas (Tzourio-Mazoyer et al. 2002).
Interregional connectivity
We performed inter-regional connectivity analyses on four distinct ROIs identified as showing a significant condition difference in the source localization analyses. TFRs of the complex Fourier spectra for each trial at each ROI was obtained by using a sliding Hanning tapers with an adaptive time window of three cycles for each frequency (ΔT = 3/f). We calculated the inter-regional phase-locked indices of the oscillatory activity (2–30 Hz) between the four ROIs (creating six inter-regional connections) for the time period of interest (−0.5 s to 1 s of the epoch) separately for the sentence condition and wordlist condition (following Lachaux et al. 1999; Bastos and Schoffelen 2016). The phase locking index (PLI) is calculated across all trials for each condition, frequency and time point. We used the following formula to calculate the PLI:
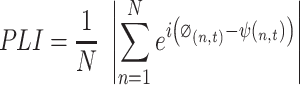 |
Applied to our study, the PLI reflects the consistency of the phase difference of oscillatory activity across trials between 2 ROIs. Here: N is the number of trials; ∅(n,t) is the phase (obtained from the complex spectra) at time t in trial n in one ROI; and ψ(n,t) is the phase at time t in trial n in the other ROI. A PLI of 0 indicates no phase locking between the activities of the 2 ROIs, whereas a PLI of 1 is indicative of perfect phase locking.
We then performed cluster-level randomization tests, involving 1000 permutations, (Maris and Oostenveld 2007) on the PLIs in order to identify the inter-regional connections in which there was a significant difference in phase-locking between the 2 experimental conditions (for a similar statistical approach see, Schmidt et al. 2014). The use of a cluster-level randomization test, in which a Monte Carlo p-value is obtained, enables the control of multiple comparisons of different ROIs and time-frequency points. To correct for the multiple analyses performed across the six different inter-regional connections, we applied a Bonferroni correction (α/n comparisons) to our critical p value of interest (.05/6 = 0.0083). We used the same pre-defined frequency bands as for the oscillatory time-frequency analyses: theta (4–7 Hz); alpha (8–12 Hz); low beta (15–20 Hz) and high beta (25–30 Hz).
Results
We compared participants’ MEG activity during the comprehension of minimal sentences that were highly likely to require binding (a pronoun combined with a pseudo-verb with the corresponding morphological inflection; “she grushes”) to wordlists that did not require binding (2 pseudo-verbs; “cugged grushes”). Our findings reveal 2 key mechanisms of syntactic binding.
Less alpha power in the left-lateralized brain network when syntactic binding occurs
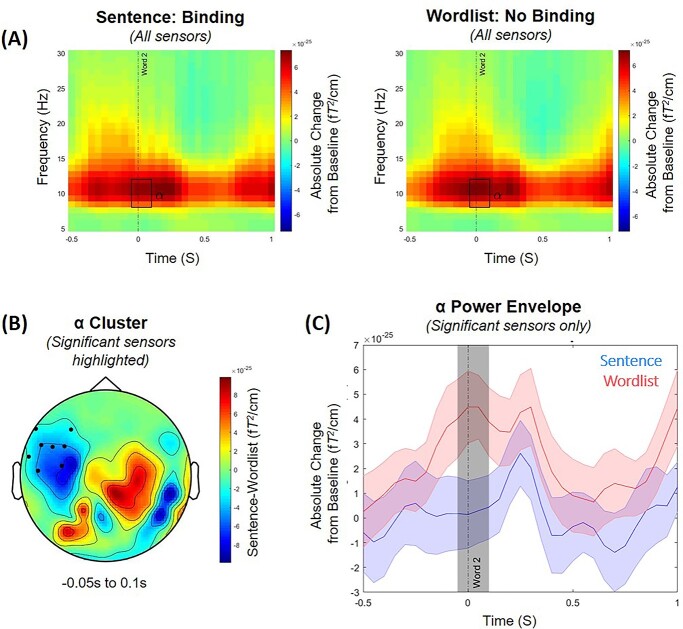
The grand-average of the TFR of power averaged across all sensors aligned to the onset of the second word are summarized in Fig. 2A for the sentence condition in which syntactic binding was highly likely to occur, and the wordlist condition in which binding was highly unlikely For TFRs of the complete epoch [−2.7 to 1.9 s], see Supplementary Materials 1. In both conditions, there are power increases in alpha and low beta surrounding the presentation of the second word (at 0 s; “grushes”). Approximately 0.5 s after the second word presentation, the strength of the alpha and low beta power signal becomes less pronounced in both conditions (although it is still positive compared with baseline).
Fig. 2.
(A) Time–frequency representations of power averaged across all sensors, expressed as an absolute change from the baseline period (i.e. −2.1 to −1.6 s before the onset of the second word) for the sentence condition (left panel) in which syntactic binding was highly likely to occur (e.g. “she grushes”), and the wordlist condition (right panel) in which binding was highly unlikely (e.g. “cugged grushes”). Time relates to the main time period of interest aligned to the onset of the second word (at 0 s). The rectangle highlights the time period where we observed the significant difference in alpha power (8–12 Hz) between the 2 conditions (−0.05 to 0.1 s; P = 0.021). (B) The scalp topography of the condition contrast (sentence minus wordlist) of the averaged alpha power activity in the time window (−0.05 to 0.1 s) where we observed the significant difference in alpha power between the 2 conditions. The black dots illustrate where this effect was largest at the scalp level. (C) The time course of the alpha power envelope for the sensors showing a significant difference in power between the sentence (blue) and wordlist (red) conditions. The shaded colored areas represent the standard error of the mean. The shaded gray area indicates the time window in which the difference between conditions is significant, centred around the presentation of the second word (−0.05 to 0.1 s).
The statistical results of the cluster-based permutation tests (which controlled for multiple comparisons of different channels and time–frequency points) of the time window of interest revealed that there was a significant condition difference in the alpha frequency range (8–12 Hz) surrounding the presentation of the second word where alpha power was lower in the sentence condition, compared with the wordlist condition (P = 0.021, see Fig. 2B and C). This difference was maximal around the onset of the second word (−0.05 to 0.1 s) over a cluster of sensors predominantly in the left-frontal region (Fig. 2B). Importantly, our observed oscillatory effect of alpha was distinct from the evoked fields as, when we analyzed the ERF-adjusted TFRs, we found the same significant alpha power cluster at −0.05 to 0.1 s (P = 0.022); see Supplementary Materials 2 for additional figures. We found no significant difference in oscillatory activity within the other analyzed frequency bands: theta (4–7 Hz), low beta (15–20 Hz), and high beta (25–30 Hz). When we analyzed the complete timeframe of the auditory stimuli presentation, the significant condition difference in alpha surrounding the second word remained significant (−0.05 to 0.1 s, P = 0.024), while we observed no other significant effects at any other time points or frequencies.
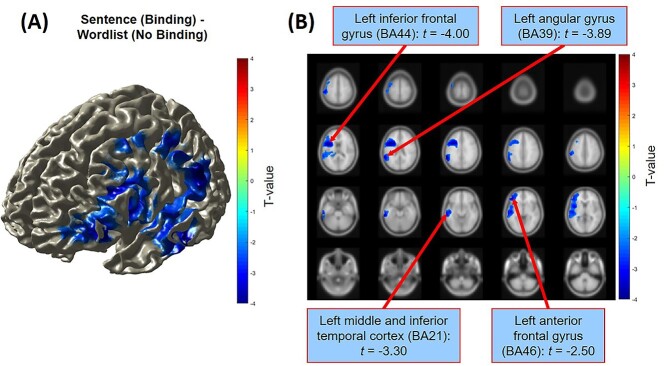
Source analyses coregistered on the participants’ anatomical MRI brain scans indicated that the significant condition difference in alpha power (8–12 Hz, −0.05 to 0.1 s) was localized to a network of left-lateralized brain regions which are typically associated with language function (Fig. 3A). Within this network, we identified 4 brain areas (see Fig. 3B) in which significant differences were found between the sentence condition and wordlist condition: the left IFG (BA44; peak coordinates [−42 7 17]); the left angular gyrus (BA39; peak coordinates [−58–48 29]); the left middle/inferior temporal cortex (BA21; peak coordinates [−60–30 -18]); and the left anterior frontal gyrus (BA46; peak coordinates [−40 43 7]).
Fig. 3.
Source localization estimates of the condition difference in alpha power (8–12 Hz) of the sentence condition (e.g. “she grushes”) minus the wordlist condition (e.g. “cugged grushes”) surrounding the presentation of the target word (−0.15 to 0.15 s) as shown for a surface (A) and sliced (B) view of the brain. The displays are masked for significant clusters only (P < 0.05). The condition difference was maximal over the left-frontal areas of the brain, with significant differences observed in the left IFG, the left angular gyrus, the left middle/inferior temporal cortex, and the left anterior frontal gyrus.
During syntactic binding, there is decreased alpha phase-locking between the left IFG and the left middle/inferior temporal cortex
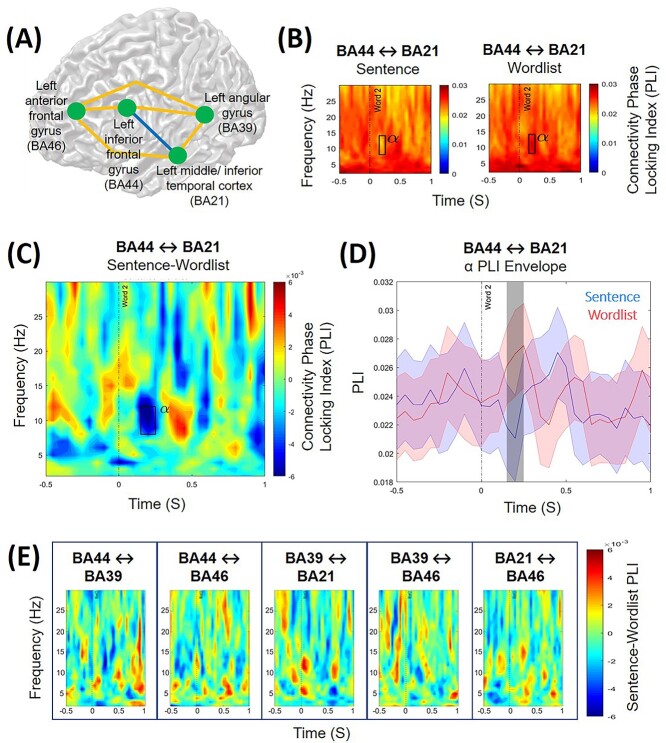
We calculated the interregional phase-locked indexes between the peak coordinates of the 4 brain areas identified as displaying significant condition differences in the source localization analyses (Fig. 4A). The statistical results of the cluster-based permutation tests (which controlled for multiple comparisons of different ROIs and time–frequency points) revealed a significant condition difference (P = 0.005) in interregional phase-locking in alpha activity (8–12 Hz) between the left IFG (BA44) and the left middle/inferior temporal cortex (BA21) following the presentation of the second word that required binding (around 0.15–0.25 s). During this time period, there was significantly less alpha phase-locking between these 2 brain regions in the sentence condition, compared with the wordlist condition (Fig. 4B–D). While we are aware that amplitude may correlate with PLI (Van Diepen and Mazaheri 2018), we consider that it is unlikely that our observed condition difference in the PLIs is driven by amplitude differences alone because it occurred at a time interval when there was not the greatest amplitude difference between the 2 conditions, and because we only observed the effect between 2 specific ROIs, not the whole analyzed left-lateralized brain network. We did not find any other significant condition differences in interregional phase-locking in the other connections between our ROIs (Fig. 4E).
Fig. 4.
(A) Interregional phase-locking differences of oscillatory activity (2–30 Hz) were calculated between 4 distinct brain regions of interest in the left hemisphere for the time period of interest centred around the presentation of the target word (−0.5 to 1 s). (B) A significant condition difference in interregional phase-locking was observed between the left interior frontal gyrus (BA44) and the left middle and inferior temporal cortex (BA21) in the alpha frequency range (8–12 Hz). Time–frequency estimates of the phase-locking index are shown for the sentence condition (“she grushes”) and the wordlist condition (“cugged grushes”). There was significantly greater phase-locking of alpha activity between BA44 and BA21 in the wordlist condition, compared with the sentence condition, 0.15–0.25 s following the presentation of the target word (P = 0.005), as highlighted by the rectangle. (C) The condition contrast (sentence minus wordlist) of the phase-locking index between BA44 and BA21. The rectangle highlights the time window and frequency range in which significantly different phase-locking was observed between the conditions. (D) The time course of the phase-locking index for the alpha frequency range (8–12 Hz) between BA44 and BA21 for the sentence condition (red) and wordlist condition (blue). The shaded colored areas represent the standard error of the mean. The shaded gray area indicates the time window in which the difference between conditions was significant (0.15–0.25 s). (E) The interregional phase-locking index of the condition contrast (sentence minus wordlist) between the other brain ROIs; no significant differences were found between the sentence and wordlist conditions.
Discussion
The findings of our MEG study suggest that minimal syntax composition is associated with distinct oscillatory changes in alpha band activity and the engagement of the left-lateralized language network of the brain. In the reported experiment, we compared minimal pseudo-verb sentences for which it was highly likely that syntactic binding may plausibly occur (e.g. “she grushes”) to wordlists for which binding was highly unlikely to occur (e.g. “cugged grushes”). We found that surrounding (−0.05 to 0.1 s) the presentation of the target word (“grushes”), alpha power was significantly less in the sentence, compared with the wordlist, condition. The sources of this condition difference were localized to left-lateralized brain regions, including the IFG, angular gyrus, middle/inferior temporal cortex, and anterior frontal gyrus. Moreover, following the presentation of the target word (0.15–0.25 s), we observed decreased alpha phase-locking between the LIFG and the left middle/inferior temporal cortex in the sentence, compared with the wordlist, condition, indicating that syntactic binding is associated with less coupling of alpha activity between these regions.
Modulation in alpha power in a network of left-lateralized language regions reflects an expectation of binding to occur
Compared with baseline, we observed an increase in alpha power in both conditions during the comprehension of the second word, but critically, this power increase was less when syntactic binding was required, compared with when no binding was required. This modulation in alpha power is consistent with existing evidence of alpha oscillatory activity in compositional processing (Lam et al. 2016; Segaert et al. 2018; Gastaldon et al. 2020) and the view that neural oscillations subserve the processing of syntactic information within language-relevant cortices (Meyer 2018; Prystauka and Lewis 2019). Our findings further demonstrate that alpha operates in auditory language processing in addition to its role in visual processing in occipital areas (Mazaheri et al. 2014; Zumer et al. 2014), supporting a varied functionality of alpha oscillations in multiple sensory systems in different cortical regions (Foxe and Snyder 2011).
We suggest that, in our experiment, the observed lesser alpha power in the binding context around the presentation of the target word reflects an expectation of binding to occur. When comprehending linguistic input, we build expectations in order to predict upcoming words (Chang et al. 2006; Kuperberg and Jaeger 2016); indeed, probability estimates are considered to be inherent within the neural linguistic system (Kutas et al. 2011). Thus, if the first word was a pronoun, as opposed to a pseudo-verb, the participant may reasonably expect that binding was likely to be required given their existing knowledge (based on language use in everyday life) about the properties and syntactic function of pronouns. This interpretation is consistent with studies that have found greater alpha suppression (i.e. decreased alpha power compared with baseline) when participants comprehend a highly predictive, compared with a less predictive, sentence (Piai et al. 2014; Rommers et al. 2017; Wang et al. 2017) and the proposed role of alpha power decreases in controlling the allocation of the brain’s resources (Jensen and Mazaheri 2010; Klimesch 2012). In particular, decreased alpha power limits cortical excitability, thereby allowing for continual processing regardless of alpha phase (medium-to-high attentional state); whereas, when alpha power is higher, cortical processing is more discontinuous as it depends on the phase of the alpha rhythm (rhythmic attentional state) (Jensen et al. 2014; Van Diepen et al. 2019; Alavash et al. 2021). Our finding of less alpha power around the presentation of the target word that required binding (compared with a no binding context) may therefore reflect the initiation of anticipatory binding processes (for which a medium-to-high attentional state is required), along with the increased engagement of brain regions involved in syntactic binding.
The observed condition difference in alpha power was localized to a left-lateralized network of brain regions, consistent with established neurobiological models of linguistic processing (Hagoort 2003; Tyler and Marslen-Wilson 2008; Friederici 2011). The implication of the LIFG (BA44) is expected given the region’s proposed role in managing the combination of words into a coherent syntactic structure (Hagoort 2005; Snijders et al. 2008; Segaert et al. 2012; Uddén et al. 2019) and in computing dependency structures (Lopopolo et al. 2021). Indeed, the LIFG has been identified in previous studies of minimal syntactic binding (Zaccarella and Friederici 2015; Zaccarella et al. 2017) and top-down predictive processing (Matchin et al. 2017; Strijkers et al. 2019). The other 3 left-lateralized regions we identified—angular gyrus (BA39), anterior frontal gyrus (BA46), and middle/inferior temporal cortex (BA21)—have also been found to contribute toward syntactic processing (Giraud 2004; Humphries et al. 2006; Menenti et al. 2011; Segaert et al. 2012; Matchin et al. 2017). Our findings therefore suggest that successful composition of the syntactic properties between words in a sentence is driven by the engagement of a distributed network of left-lateralized regions (including the frontal gyri, temporal cortex, and angular gyrus), and critically their coordination (as we discuss in more detail in the next section). Moreover, given that we did not observe any effect of compositionality in the ATL in our task (in which contributions from semantics were minimized), our findings further add to the current evidence that the functional role of the ATL relates primarily to semantic, not syntactic, composition (Del Prato and Pylkkänen 2014; Wilson et al. 2014; Kim and Pylkkänen 2019; Pylkkänen 2019).
However, our findings of alpha power decrease are somewhat at odds with Segaert et al. (2018) who, using a similar paradigm but with EEG, found syntactic binding to be associated with alpha power increases. One explanation for this difference may reflect MEG vs. EEG differences in spatial coherence and sensitivity to deeper brain tissues that can lead to differences in detectable power (Lopes da Silva 2013; Bénar et al. 2019). In particular, MEG and EEG differ in their sensitivity to the radical and tangential components of the dipolar sources in the brain, which can lead to the 2 producing diverging estimates for the same cognitive process (Edgar et al. 2003; Dehghani et al. 2010; Fonteneau et al. 2015; Ross et al. 2020). An alternative explanation for the study differences may relate to morpho-syntactic differences between English (used in this study) and Dutch (used in Segaert et al. 2018). Compared with English, Dutch is more morphologically complex in that there are a greater number of possible verb inflections (to signal its binding to a preceding pronoun) and there are more rules on inflection usage (Booij 2019). This could mean that Dutch listeners focus more on morphological congruency, whereas English speakers focus more on overall sentential composition, leading to group differences in detectable alpha power. Further work is therefore needed to uncover how differences in the complexity of the inflection system of a language may drive differences in the oscillatory signature of syntax composition even at its most basic 2-word level. Nevertheless, the findings of our study and Segaert et al. (2018) should not be considered as directly opposed, but instead, reflective of the different components of the wider neural processes involved in syntax composition (the intricacies of which may differ between languages or which may be differently detected by MEG vs. EEG), and the more general functionality of alpha oscillations in syntax processing (Meyer 2018; Prystauka and Lewis 2019).
Decreased alpha band phase-locking in the language network reflects strengthened network communication required for successful syntactic binding
Interregional connectivity analyses revealed that syntactic binding was associated with less coupling of alpha between the LIFG and the left middle/inferior temporal cortex: we found less alpha phase-locking in the sentence, compared with the wordlist, condition following the presentation of the target word (0.15–0.25 s). The time window of the effect, after the auditory processing of the target word (which typically occurs within the first 0.1 s; Zouridakis et al. 1998), suggests that it reflects the underlying syntax composition mechanisms taking place as opposed to an expectation of binding to occur. Our finding is consistent with evidence that less alpha band coupling (also referred to as alpha desynchronization) between relevant brain regions is beneficial for language comprehension and can predict successful sentence encoding (Becker et al. 2013; Magazzini et al. 2016; Vassileiou et al. 2018; Alavash et al. 2021). This functional connectivity reflects the dynamic interaction among distributed brain regions that are subserved by deep white matter pathways and which communicate through frequency-specific networks (Schoffelen et al. 2017; White et al. 2018; Sarubbo et al. 2020). This communication may operate through top-down mechanisms of cortical disinhibition, such that alpha band desynchronization serves to functionally disinhibit the cortex to enable information to be transferred from and to specific areas for processing (Jensen and Mazaheri 2010; Klimesch 2012; Sadaghiani and Kleinschmidt 2016).
We suggest that similar mechanisms of frequency-specific communication and alpha-driven disinhibition were operating in our task when participants comprehended sentences that needed binding (“she grushes”). In order to bind the 2 words together into a minimal syntactic structure, increased information, such as the labels of syntactic components (Murphy 2015), needed to be transferred between the LIFG and the middle/inferior temporal cortex. Thus, the less alpha band synchronization between these 2 regions, the greater the cortical disinhibition; this, in turn, strengthens the communication within the cortical and oscillatory network involved in syntax composition, thereby enhancing participants’ processing.
Literature suggests that an increase in alpha activity in a brain area reflects gating of information in that area (Klimesch et al. 2007; Jensen and Mazaheri 2010; Scheeringa et al. 2011; Van Diepen et al. 2019). The precise mechanism underlying this inhibition has not yet been fully elucidated, though one proposition is that an alpha cycle may reflect pulses that could be inhibiting the firing rate of neurons (Haegens et al. 2011). We ourselves have previously speculated that alpha activity can be viewed as rhythmic pulses of inhibition and excitability that cycle on and off approximately every 100 ms (Mazaheri and Jensen 2010). Within a region, alpha activity can be seen as serving as “gain-control” by limiting the duty-cycle (i.e. the ratio between on/off) of information processing in a cortical region (Spaak et al. 2012). Based on Van Diepen et al.'s (2019) recent model of alpha synchronization, we speculate that in a situation where alpha activity is synchronous between 2 brain areas, information flow between them is gated because of the restriction posed by the duty cycle of processing; however, a suppression of alpha activity (i.e. decrease in synchrony between brain areas) would correspond to the lifting of any constraints of information flow between the 2 areas. One caveat here is that the empirical work supporting such a model has come from simple visual attention tasks, and more work needs to be done in order to get a clearer view on the role of alpha modulation in more complex cognitive processes such as language. Nevertheless, based on this understanding, our findings are indicative of a top-down downregulation of alpha oscillations that operate as part of a broader attentional control network throughout the brain’s sensory channels, which during speech comprehension act to promote the spread of information between language relevant cortices (Sadaghiani and Kleinschmidt 2016; Alavash et al. 2021). This interpretation further fits with the wider understanding of the role of alpha oscillations in controlling the access, transfer, and storage of information within the brain (Klimesch 2012; Bonnefond et al. 2017).
Mechanistically, we tentatively suggest that there is a link between the earlier decreased alpha power (observed around the onset of the second word) and the later decreased alpha phase-locking, with each reflecting a complimentary but distinct part of the neural syntax composition process. However, in order to confirm and strengthen this interpretation, future research with a design utilizing more trials (i.e. to assess phase-locking in low alpha power vs. high alpha power trials) and more participants is required. With regard to the latter point, to explore correlations at the participant level between these 2 observed effects, a considerably large sample size would be required (recommended at least 80 participants for a stable correlation estimate in neuroimaging research; Grady et al. 2021). Addressing this question would further enhance the understanding of syntax composition in the brain.
Summary
In sum, when comparing minimal pseudo-verb sentences for which it was highly likely that syntactic binding may plausibly occur (“she grushes”) to wordlists for which binding was highly unlikely to occur (“cugged grushes”), we first found evidence of less alpha power in left-lateralized brain regions; we suggest that this reflects an expectation of binding to occur. Second, we found that during syntactic binding, there was decreased alpha phase-locking between the LIFG and the left middle/inferior temporal cortex; we suggest that this results from increased information transfer and the strengthening of the neural network involved in syntax composition. The observed oscillatory modulations (including decreased power and interregional coupling) occurred in brain regions in the left hemisphere known to be relevant for language processing. These regions are not uniquely selective for syntactic processing; rather, we suggest that each region plays a contributing role in syntax composition mechanisms and that coordination between these regions (particularly the LIFG and left middle/inferior temporal cortex) is critical for successful syntactic binding. Altogether, our findings contribute to the wider understanding of the rapid spatial–temporal dynamics of syntactic processing in the brain that occur independently of semantic processing; we suggest that future research builds on this by exploring the mechanistic links between different oscillatory characteristics of the syntactic binding process.
Supplementary Material
Acknowledgments
We thank Jonathon Winter and Nina Salman for their help with data collection.
Contributor Information
Sophie M Hardy, Centre for Human Brain Health, University of Birmingham, Birmingham B15 2TT, UK; Department of Psychology, University of Warwick, Coventry CV4 7AL, UK.
Ole Jensen, Centre for Human Brain Health, University of Birmingham, Birmingham B15 2TT, UK.
Linda Wheeldon, Department of Foreign Languages and Translations, University of Agder, Kristiansand 4630, Norway.
Ali Mazaheri, Centre for Human Brain Health, University of Birmingham, Birmingham B15 2TT, UK; School of Psychology, University of Birmingham, Birmingham B15 2TT, UK.
Katrien Segaert, Centre for Human Brain Health, University of Birmingham, Birmingham B15 2TT, UK; School of Psychology, University of Birmingham, Birmingham B15 2TT, UK.
Funding
This research was supported by an Economic and Social Research Council (ESRC) studentship awarded to SMH from the University of Birmingham Doctoral Training Centre (grant number: ES/J50001X/1). This publication was further made possible through the support of grants awarded to OJ from the Wellcome Trust (Investigator Award in Science; grant number 207550) and the Royal Society (Wolfson Research Merit Award).
Conflict of interest statement. The authors report no potential conflict of interest.
References
- Alavash M, Tune S, Obleser J. Dynamic large-scale connectivity of intrinsic cortical oscillations supports adaptive listening in challenging conditions. PLoS Biol. 2021:19(10):e3001410. 10.1371/journal.pbio.3001410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastiaansen M, Hagoort P. Frequency-based segregation of syntactic and semantic unification during online sentence level language comprehension. J Comput Neurosci. 2015:27(11):2095–2107. 10.1162/jocn_a_00829. [DOI] [PubMed] [Google Scholar]
- Bastiaansen M, Magyari L, Hagoort P. Syntactic unification operations are reflected in oscillatory dynamics during on-line sentence comprehension. J Cogn Neurosci. 2010:22(7):1333–1347. 10.1162/jocn.2009.21283. [DOI] [PubMed] [Google Scholar]
- Bastos AM, Schoffelen J-M. A tutorial review of functional connectivity analysis methods and their interpretational pitfalls. Front Syst Neurosci. 2016:9:175. https://www.frontiersin.org/articles/10.3389/fnsys.2015.00175/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker R, Pefkou M, Michel CM, Hervais-Adelman AG. Left temporal alpha-band activity reflects single word intelligibility. Front Syst Neurosci. 2013:7:121. https://www.frontiersin.org/articles/10.3389/fnsys.2013.00121/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bemis DK, Pylkkänen L. Simple composition: a magnetoencephalography investigation into the comprehension of minimal linguistic phrases. J Neurosci. 2011:31(8):2801–2814. 10.1523/jneurosic.5003-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bemis DK, Pylkkänen L. Basic linguistic composition recruits the left anterior temporal lobe and left angular gyrus during both listening and reading. Cereb Cortex. 2013:23(8):1859–1873. 10.1093/cercor/bhs170. [DOI] [PubMed] [Google Scholar]
- Bénar CG, Grova C, Jirsa VK, Lina JM. Differences in MEG and EEG power-law scaling explained by a coupling between spatial coherence and frequency: a simulation study. J Comput Neurosci. 2019:47(1):31–41. 10.1007/s10827-019-00721-9. [DOI] [PubMed] [Google Scholar]
- Besl PJ, McKay ND. A method for registration of 3-D shapes. IEEE Trans Pattern Anal Mach Intell. 1992:14(2):239–256. 10.1109/34.121791. [DOI] [Google Scholar]
- Blanco-Elorrieta E, Ferreira VS, Del Prato P, Pylkkänen L. The priming of basic combinatory responses in MEG. Cognition. 2018:170:49–63. https://www.sciencedirect.com/science/article/pii/S0010027717302536?via%3Dihub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boersma P. PRAAT, a system for doing phonetics by computer. Glot Intl. 2001:5(9):341–345. [Google Scholar]
- Bonnefond M, Kastner S, Jensen O. Communication between brain areas based on nested oscillations. eNeuro. 2017:4(2):e0153. 10.1523/ENEURO.0153-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booij G. The morphology of Dutch. Oxford (UK): Oxford University Press; 2019 [Google Scholar]
- Chang F, Dell GS, Bock K. Becoming syntactic. Psychol Rev. 2006:113(2):234–272. 10.1037/0033-295X.113.2.234. [DOI] [PubMed] [Google Scholar]
- Chomsky N. The minimalist program. Cambridge (MA): The MIT Press; 1995. 10.1017/S0022226797006889 [DOI] [Google Scholar]
- Dehghani N, Cash SS, Chen CC, Hagler DJ, Huang M, Dale AM, Halgren E. Divergent cortical generators of MEG and EEG during human sleep spindles suggested by distributed source modeling. PLoS One. 2010:5(7):e11454. 10.1371/journal.pone.0011454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Prato P, Pylkkänen L. MEG evidence for conceptual combination but not numeral quantification in the left anterior temporal lobe during language production. Front Psychol. 2014:5:524. https://www.frontiersin.org/articles/10.3389/fpsyg.2014.00524/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar JC, Huang MX, Weisend MP, Sherwood A, Miller GA, Adler LE, Cañive JM. Interpreting abnormality: an EEG and MEG study of P50 and the auditory paired-stimulus paradigm. Biol Psychol. 2003:65(1):1–20. 10.1016/S0301-0511(03)00094-2. [DOI] [PubMed] [Google Scholar]
- Fonteneau E, Bozic M, Marslen-Wilson WD. Brain network connectivity during language comprehension: interacting linguistic and perceptual subsystems. Cereb Cortex. 2015:25(10):3962–3976. 10.1093/cercor/bhu283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxe JJ, Snyder AC. The role of alpha-band brain oscillations as a sensory suppression mechanism during selective attention. Front Psychol. 2011:2. https://www.frontiersin.org/articles/10.3389/fpsyg.2011.00154/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici AD. The brain basis of language processing: from structure to function. Physiol Rev. 2011:91(4):1357–1392. 10.1152/physrev.00006.2011. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Meyer M, Von Cramon DY. Auditory language comprehension: an event-related fMRI study on the processing of syntactic and lexical information. Brain Lang. 2000:74(2):289–300. 10.1006/brln.2000.2313. [DOI] [PubMed] [Google Scholar]
- Fyshe A, Sudre G, Wehbe L, Rafidi N, Mitchell TM. The lexical semantics of adjective-noun phrases in the human brain. Hum Brain Mapp. 2019:40(15):4457–4469. 10.1002/hbm.24714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gastaldon S, Arcara G, Navarrete E, Peressotti F. Commonalities in alpha and beta neural desynchronizations during prediction in language comprehension and production. Cortex. 2020:133:328–345. https://www.sciencedirect.com/science/article/abs/pii/S0010945220303701?via%3Dihub. [DOI] [PubMed] [Google Scholar]
- Giraud AL. Contributions of sensory input, auditory search and verbal comprehension to cortical activity during speech processing. Cereb Cortex. 2004:14(3):247–255. 10.1093/cercor/bhg124. [DOI] [PubMed] [Google Scholar]
- Grady CL, Rieck JR, Nichol D, Rodrigue KM, Kennedy KM. Influence of sample size and analytic approach on stability and interpretation of brain-behavior correlations in task-related fMRI data. Hum Brain Mapp. 2021:42(1):204–219. 10.1002/hbm.25217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J, Kujala J, Hamalainen M, Timmermann L, Schnitzler A, Salmelin R. Dynamic imaging of coherent sources: studying neural interactions in the human brain. Proc Natl Acad Sci. 2001:98(2):694–699. 10.1073/pnas.98.2.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegens S, Nácher V, Luna R, Romo R, Jensen O. α-Oscillations in the monkey sensorimotor network influence discrimination performance by rhythmical inhibition of neuronal spiking. Proc Natl Acad Sci. 2011:108(48):19377–19382. 10.1073/pnas.1117190108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagoort P. How the brain solves the binding problem for language: a neurocomputational model of syntactic processing. NeuroImage. 2003:20:S18–S29. https://www.sciencedirect.com/science/article/abs/pii/S1053811903005251?via%3Dihub. [DOI] [PubMed] [Google Scholar]
- Hagoort P. On Broca, brain, and binding: a new framework. Trends Cogn Sci. 2005:9(9):416–423. 10.1016/j.tics.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Hagoort P. The neurobiology of language beyond single-word processing. Science. 2019:366(6461):55–58. 10.1126/science.aax0289. [DOI] [PubMed] [Google Scholar]
- Hald LA, Bastiaansen MCM, Hagoort P. EEG theta and gamma responses to semantic violations in online sentence processing. Brain Lang. 2006:96(1):90–105. 10.1016/j.bandl.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Humphries C, Binder JR, Medler DA, Liebenthal E. Syntactic and semantic modulation of neural activity during auditory sentence comprehension. J Cogn Neurosci. 2006:18(4):665–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen O, Mazaheri A. Shaping functional architecture by oscillatory alpha activity: gating by inhibition. Front Hum Neurosci. 2010:4:186. https://www.sciencedirect.com/science/article/abs/pii/S1053811903005251?via%3Dihub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen O, Gips B, Bergmann TO, Bonnefond M. Temporal coding organized by coupled alpha and gamma oscillations prioritize visual processing. Trends Neurosci. 2014:37(7):357–369. 10.1016/j.tins.2014.04.001. [DOI] [PubMed] [Google Scholar]
- Kim S, Pylkkänen L. Composition of event concepts: evidence for distinct roles for the left and right anterior temporal lobes. Brain Lang. 2019:188:18–27. https://www.sciencedirect.com/science/article/pii/S0093934X18301330. [DOI] [PubMed] [Google Scholar]
- Klimesch W. Alpha-band oscillations, attention, and controlled access to stored information. Trends Cogn Sci. 2012:16(12):606–617. 10.1016/j.tics.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W, Sauseng P, Hanslmayr S. EEG alpha oscillations: the inhibition-timing hypothesis. Brain Res Rev. 2007:53(1):63–88. 10.1016/j.brainresrev.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Kochari AR, Lewis AG, Schoffelen J-M, Schriefers H. Semantic and syntactic composition of minimal adjective-noun phrases in Dutch: an MEG study. Neuropsychologia. 2021:155:107754. 10.1016/j.neuropsychologia.2021.107754. [DOI] [PubMed] [Google Scholar]
- Kuperberg GR, Jaeger TF. What do we mean by prediction in language comprehension? Lang Cogn Neurosci. 2016:31(1):32–59. 10.1080/23273798.2015.1102299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutas M, DeLong KA, Smith NJ. A look around at what lies ahead: prediction and predictability in language processing. In: Bar M, editors. Predictions in the brain: using our past to generate a future. Oxford (UK): Oxford University Press; 2011. pp. 190–207 [Google Scholar]
- Lachaux J-P, Rodriguez E, Martinerie J, Varela FJ. Measuring phase synchrony in brain signals. Hum Brain Mapp. 1999:8(4):194–208. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam NHL, Schoffelen JM, Uddén J, Hultén A, Hagoort P. Neural activity during sentence processing as reflected in theta, alpha, beta, and gamma oscillations. NeuroImage. 2016:142:43–54. https://www.sciencedirect.com/science/article/abs/pii/S1053811916002032?via%3Dihub. [DOI] [PubMed] [Google Scholar]
- Lopes da Silva F. EEG and MEG: relevance to neuroscience. Neuron. 2013:80(5):1112–1128. 10.1016/j.neuron.2013.10.017. [DOI] [PubMed] [Google Scholar]
- Lopopolo A, van den Bosch A, Petersson K-M, Willems RM. Distinguishing syntactic operations in the brain: dependency and phrase-structure parsing. Neurobiol Lang. 2021:2(1):152–175. 10.1162/nol_a_00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor LJ, Pulvermüller F, van Casteren M, Shtyrov Y. Ultra-rapid access to words in the brain. Nat Commun. 2012:3(1):711. 10.1038/ncomms1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magazzini L, Ruhnau P, Weisz N. Alpha suppression and connectivity modulations in left temporal and parietal cortices index partial awareness of words. NeuroImage. 2016:133:279–287. https://www.sciencedirect.com/science/article/pii/S1053811916002305?via%3Dihub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maris E, Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods. 2007:164(1):177–190. 10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Matchin W, Hickok G. The cortical organization of syntax. Cereb Cortex. 2020:30(3):1481–1498. 10.1093/cercor/bhz180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matchin W, Hammerly C, Lau E. The role of the IFG and pSTS in syntactic prediction: evidence from a parametric study of hierarchical structure in fMRI. Cortex. 2017:88:106–123. https://www.sciencedirect.com/science/article/abs/pii/S0010945216303549?via%3Dihub. [DOI] [PubMed] [Google Scholar]
- Mazaheri A, Jensen O. Rhythmic pulsing: linking ongoing brain activity with evoked responses. Front Hum Neurosci. 2010:4:177. https://linkinghub.elsevier.com/retrieve/pii/S0926641004003519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazaheri A, Picton TW. EEG spectral dynamics during discrimination of auditory and visual targets. Cogn Brain Res. 2005:24(1):81–96. 10.1016/j.cogbrainres.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Mazaheri A, Nieuwenhuis ILC, Van Dijk H, Jensen O. Prestimulus alpha and mu activity predicts failure to inhibit motor responses. Hum Brain Mapp. 2009:30(6):1791–1800. 10.1002/hbm.20763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazaheri A, van Schouwenburg MR, Dimitrijevic A, Denys D, Cools R, Jensen O. Region-specific modulations in oscillatory alpha activity serve to facilitate processing in the visual and auditory modalities. NeuroImage. 2014:87:356–362. https://www.sciencedirect.com/science/article/abs/pii/S1053811913010756?via%3Dihub. [DOI] [PubMed] [Google Scholar]
- Menenti L, Gierhan SME, Segaert K, Hagoort P. Shared language: overlap and segregation of the neuronal infrastructure for speaking and listening revealed by functional MRI. Psychol Sci. 2011:22(9):1173–1182. 10.1177/0956797611418347. [DOI] [PubMed] [Google Scholar]
- Meyer L. The neural oscillations of speech processing and language comprehension: state of the art and emerging mechanisms. Eur J Neurosci. 2018:48(7):2609–2621. 10.1111/ejn.13748. [DOI] [PubMed] [Google Scholar]
- Meyer L, Obleser J, Friederici AD. Left parietal alpha enhancement during working memory-intensive sentence processing. Cortex. 2013:49(3):711–721. 10.1016/j.cortex.2012.03.006. [DOI] [PubMed] [Google Scholar]
- Murphy E. The brain dynamics of linguistic computation. Front Psychol. 2015:6:1515. https://www.frontiersin.org/articles/10.3389/fpsyg.2015.01515/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolte G. The magnetic lead field theorem in the quasi-static approximation and its use for magnetoencephalography forward calculation in realistic volume conductors. Phys Med Biol. 2003:48(22):3637–3652. 10.1088/0031-9155/48/22/002. [DOI] [PubMed] [Google Scholar]
- Oostenveld R, Fries P, Maris E, Schoffelen JM. FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci. 2011:2011:156869. https://www.hindawi.com/journals/cin/2011/156869/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallier C, Devauchelle AD, Dehaene S. Cortical representation of the constituent structure of sentences. Proc Natl Acad Sci. 2011:108(6):2522–2527. 10.1073/pnas.1018711108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piai V, Roelofs A, Maris E. Oscillatory brain responses in spoken word production reflect lexical frequency and sentential constraint. Neuropsychologia. 2014:53(1):146–156. 10.1016/j.neuropsychologia.2013.11.014. [DOI] [PubMed] [Google Scholar]
- Poulisse C, Wheeldon L, Segaert K. Evidence against preserved syntactic comprehension in healthy aging. J Exp Psychol Learn Mem Cogn. 2019:45(12):2290–2308. https://www.sciencedirect.com/science/article/abs/pii/S0028393220301962?via%3Dihub. [DOI] [PubMed] [Google Scholar]
- Poulisse C, Wheeldon L, Limachya R, Mazaheri A, Segaert K. The oscillatory mechanisms associated with syntactic binding in healthy ageing. Neuropsychologia. 2020:146:107523. 10.1016/j.neuropsychologia.2020.107523. [DOI] [PubMed] [Google Scholar]
- Prystauka Y, Lewis AG. The power of neural oscillations to inform sentence comprehension: a linguistic perspective. Lang Linguist Compass. 2019:13(9):e12347. 10.1111/lnc3.12347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pylkkänen L. The neural basis of combinatory syntax and semantics. Science. 2019:366(6461):62–66. 10.1126/science.aax0050. [DOI] [PubMed] [Google Scholar]
- Pylkkänen L, Bemis DK, Blanco-Elorrieta E. Building phrases in language production: an MEG study of simple composition. Cognition. 2014:133(2):371–384. 10.1016/j.cognition.2014.07.001. [DOI] [PubMed] [Google Scholar]
- Roland D, Dick F, Elman JL. Frequency of basic English grammatical structures: a corpus analysis. J Mem Lang. 2007:57(3):348–379. 10.1016/j.jml.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommers J, Dickson DS, Norton JJS, Wlotko EW, Federmeier KD. Alpha and theta band dynamics related to sentential constraint and word expectancy. Lang Cogn Neurosci. 2017:32(5):576–589. 10.1080/23273798.2016.1183799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross B, Tremblay KL, Alain C. Simultaneous EEG and MEG recordings reveal vocal pitch elicited cortical gamma oscillations in young and older adults. NeuroImage. 2020:204:116253. 10.1016/j.neuroimage.2019.116253. [DOI] [PubMed] [Google Scholar]
- Sadaghiani S, Kleinschmidt A. Brain networks and α-oscillations: structural and functional foundations of cognitive control. Trends Cogn Sci. 2016:20(11):805–817. 10.1016/j.tics.2016.09.004. [DOI] [PubMed] [Google Scholar]
- Sarubbo S, Tate M, De Benedictis A, Merler S, Moritz-Gasser S, Herbet G, Duffau H. Mapping critical cortical hubs and white matter pathways by direct electrical stimulation: an original functional atlas of the human brain. NeuroImage. 2020:205:116237. https://www.sciencedirect.com/science/article/pii/S1053811919308286?via%3Dihub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheeringa R, Mazaheri A, Bojak I, Norris DG, Kleinschmidt A. Modulation of visually evoked cortical fMRI responses by phase of ongoing occipital alpha oscillations. J Neurosci. 2011:31(10):3813–3820. 10.1523/JNEUROSCI.4697-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell M, Zaccarella E, Friederici AD. Differential cortical contribution of syntax and semantics: an fMRI study on two-word phrasal processing. Cortex. 2017:96:105–120. 10.1016/j.cortex.2017.09.002. [DOI] [PubMed] [Google Scholar]
- Schmidt BT, Ghuman AS, Huppert TJ. Whole brain functional connectivity using phase locking measures of resting state magnetoencephalography. Front Neurosci. 2014:8:141. https://www.frontiersin.org/articles/10.3389/fnins.2014.00141/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider W, Eschman A, Zuccolotto A. E-prime user’s guide. Pittsburgh (PA): Psychology Software Tools Inc; 2002 [Google Scholar]
- Schoffelen J-M, Hultén A, Lam N, Marquand AF, Uddén J, Hagoort P. Frequency-specific directed interactions in the human brain network for language. Proc Natl Acad Sci. 2017:114(30):8083–8088. 10.1073/pnas.1703155114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segaert K, Menenti L, Weber K, Petersson KM, Hagoort P. Shared syntax in language production and language comprehension - an fMRI study. Cereb Cortex. 2012:22(22):1662–1670. 10.1093/cercor/bhr249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segaert K, Mazaheri A, Hagoort P. Binding language: structuring sentences through precisely timed oscillatory mechanisms. Eur J Neurosci. 2018:48(7):2651–2662. 10.1111/ejn.13816. [DOI] [PubMed] [Google Scholar]
- Snijders TM, Vosse T, Kempen G, Van Berkum JJA, Petersson KM, Hagoort P. Retrieval and unification of syntactic structure in sentence comprehension: an fMRI study using word-category ambiguity. Cereb Cortex. 2008:19(7):1493–1503. 10.1093/cercor/bhn187. [DOI] [PubMed] [Google Scholar]
- Spaak E, Bonnefond M, Maier A, Leopold DA, Jensen O. Layer-specific entrainment of γ-band neural activity by the α rhythm in monkey visual cortex. Curr Biol. 2012:22(24):2313–2318. 10.1016/j.cub.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strijkers K, Chanoine V, Munding D, Dubarry A-S, Trébuchon A, Badier J-M, Alario F-X. Grammatical class modulates the (left) inferior frontal gyrus within 100 milliseconds when syntactic context is predictive. Sci Rep. 2019:9(1):4830. 10.1038/s41598-019-41376-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler LK, Marslen-Wilson W. Fronto-temporal brain systems supporting spoken language comprehension. Philos Trans R Soc Lond Ser B Biol Sci. 2008:363(1493):1037–1054. 10.1098/rstb.2007.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002:15(1):273–289. 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Uddén J, Hultén A, Schoffelen J-M, Lam N, Harbusch K, van den Bosch A, Kempen G, Petersson KM, Hagoort P. Supramodal sentence processing in the human brain: fMRI evidence for the influence of syntactic complexity in more than 200 participants. bioRxiv. 2019. 10.1101/5767692019 March 14 preprint: not peer reviewed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullman MT, Corkin S, Coppola M, Hickok G, Growdon JH, Koroshetz WJ, Pinker S. A neural dissociation within language: evidence that the mental dictionary is part of declarative memory, and that grammatical rules are processed by the procedural system. J Cogn Neurosci. 1997:9(2):266–276. 10.1162/jocn.1997.9.2.266. [DOI] [PubMed] [Google Scholar]
- Van Diepen RM, Mazaheri A. Cross-sensory modulation of alpha oscillatory activity: suppression, idling, and default resource allocation. Eur J Neurosci. 2017:45(11):1431–1438. 10.1111/ejn.13570. [DOI] [PubMed] [Google Scholar]
- Van Diepen RM, Mazaheri A. The caveats of observing inter-trial phase-coherence in cognitive neuroscience. Sci Rep. 2018:8(1):2990. 10.1038/s41598-018-20423-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Diepen RM, Foxe JJ, Mazaheri A. The functional role of alpha-band activity in attentional processing: the current zeitgeist and future outlook. Curr Opin Psychol. 2019:29:229–238. https://www.sciencedirect.com/science/article/pii/S2352250X1830229X?via%3Dihub. [DOI] [PubMed] [Google Scholar]
- Vassileiou B, Meyer L, Beese C, Friederici AD. Alignment of alpha-band desynchronization with syntactic structure predicts successful sentence comprehension. NeuroImage. 2018:175:286–296. https://linkinghub.elsevier.com/retrieve/pii/S1053811918302969. [DOI] [PubMed] [Google Scholar]
- Wang L, Jensen O, Van den Brink D, Weder N, Schoffelen JM, Magyari L, Hagoort P, Bastiaansen M. Beta oscillations relate to the N400m during language comprehension. Hum Brain Mapp. 2012:33(12):2898–2912. 10.1002/hbm.21410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Hagoort P, Jensen O. Language prediction is reflected by coupling between frontal gamma and posterior alpha oscillations. J Cogn Neurosci. 2017:30(3):432–447. 10.1162/jocn_a_01190. [DOI] [PubMed] [Google Scholar]
- Westerlund M, Kastner I, Al Kaabi M, Pylkkänen L. The LATL as locus of composition: MEG evidence from English and Arabic. Brain Lang. 2015:141:124–134. 10.1016/j.bandl.2014.12.003. [DOI] [PubMed] [Google Scholar]
- White EJ, Nayman C, Dunkley BT, Keller AE, Valiante TA, Pang EW. Addressing the language binding problem with dynamic functional connectivity during meaningful spoken language comprehension. Front Psychol. 2018:9:1960. 10.3389/fpsyg.2018.01960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmarsh S, Nieuwenhuis ILC, Barendregt HP, Jensen O. Sensorimotor alpha activity is modulated in response to the observation of pain in others. Front Hum Neurosci. 2011:5:91. https://www.frontiersin.org/articles/10.3389/fnhum.2011.00091/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SM, DeMarco AT, Henry ML, Gesierich B, Babiak M, Mandelli ML, Miller BL, Gorno-Tempini ML. What role does the anterior temporal lobe play in sentence-level processing? Neural correlates of syntactic processing in semantic variant primary progressive aphasia. J Cogn Neurosci. 2014:26(5):970–985. 10.1162/jocn_a_00550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaccarella E, Friederici AD. Merge in the human brain: a sub-region based functional investigation in the left pars opercularis. Front Psychol. 2015:6:1818. 10.3389/fpsyg.2015.01818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaccarella E, Meyer L, Makuuchi M, Friederici AD. Building by syntax: the neural basis of minimal linguistic structures. Cereb Cortex. 2017:27(1):411–421. 10.1093/cercor/bhv234. [DOI] [PubMed] [Google Scholar]
- Zhang L, Pylkkänen L. The interplay of composition and concept specificity in the left anterior temporal lobe: an MEG study. NeuroImage. 2015:111:228–240. 10.1016/j.neuroimage.2015.02.028. [DOI] [PubMed] [Google Scholar]
- Zhang L, Pylkkänen L. Composing lexical versus functional adjectives: evidence for uniformity in the left temporal lobe. Psychon Bull Rev. 2018:25(6):2309–2322. 10.3758/s13423-018-1469-y. [DOI] [PubMed] [Google Scholar]
- Ziegler J, Pylkkänen L. Scalar adjectives and the temporal unfolding of semantic composition: an MEG investigation. Neuropsychologia. 2016:89:161–171. https://www.sciencedirect.com/science/article/abs/pii/S0028393216302093?via%3Dihub. [DOI] [PubMed] [Google Scholar]
- Zouridakis G, Simos PG, Papanicolaou AC. Multiple bilaterally asymmetric cortical sources account for the auditory N1m component. Brain Topogr. 1998:10(3):183–189. 10.1023/A:1022246825461. [DOI] [PubMed] [Google Scholar]
- Zumer JM, Scheeringa R, Schoffelen J-M, Norris DG, Jensen O. Occipital alpha activity during stimulus processing gates the information flow to object-selective cortex. PLoS Biol. 2014:12(10):e1001965. 10.1371/journal.pbio.1001965. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.