Abstract
Neutrophils, the most abundant of all leucocytes and the first cells to arrive at the sites of sterile inflammation/injury act as a double-edged sword. On one hand, they inflict a significant collateral damage to the tissues and on the other hand, they help facilitate wound healing by a number of mechanisms. Recent studies have drastically changed the perception of neutrophils from being simple one-dimensional cells with an unrestrained mode of action to a cell type that display maturity and complex behaviour. It is now recognized that neutrophils are transcriptionally active and respond to plethora of signals by deploying a wide variety of cargo to influence the activity of other cells in the vicinity. Neutrophils can regulate macrophage behaviour, display innate immune memory, and play a major role in the resolution of inflammation in a context-dependent manner. In this review, we provide an update on the factors that regulate neutrophil production and the emerging dichotomous role of neutrophils in the context of cardiovascular diseases, particularly in atherosclerosis and the ensuing complications, myocardial infarction, and heart failure. Deciphering the complex behaviour of neutrophils during inflammation and resolution may provide novel insights and in turn facilitate the development of potential therapeutic strategies to manage cardiovascular disease.
Keywords: Neutrophils, Inflammation, Myeolopoiesis, Atherosclerosis, Myocardial infarction
1. Introduction
Human evolution selecting for reproductive and survival fitness over the millennia has led to the development of an immune system that can resist infection, prevent bleeding out due to injury, and survive starvation. Unfortunately, evolution has not prepared the human immune system for an over-fed sedentary lifestyle of the modern world. The inflammatory response initiated by microbial pathogens can also be activated by metabolic stressors during cardiovascular disease (CVD) conditions, leading to sterile inflammation. This low-grade inflammation leads to CVD, which arguably, is the foremost cause of death in the modern world. Emerging evidence attests to an important role for neutrophils in the development of coronary artery disease (CAD) and even more so during its ensuing complications, such as acute coronary syndrome (ACS) and heart failure (HF). Both clinical and experimental studies have convincingly demonstrated the presence of neutrophils in early aortic lesions and in rupture/erosion prone atherosclerotic plaques.1 Thus, neutrophil count is considered an independent prognostic factor in ACS patients, both at the time of admission and after revascularization.2 Indeed the number of circulating neutrophils is directly related to infarct size and a decline in left ventricular ejection fraction (LVEF).3 A positive correlation between plasma levels of neutrophil secretory proteins and CAD has also been established, suggesting that neutrophil-derived mediators possess detrimental effects in CAD. However, almost all anti-neutrophil strategies aimed at directly reducing their number or function have failed to produce desirable clinical benefits.4 Many reasons have been attributed to this failure ranging from poor efficacy to improper dosing, duration of treatment and the presence of concurrent risk factors.4 For the last few years, accumulating evidence suggests a role for neutrophils in the activation of reparative process for healing after an injury. From the perspective of developing new therapeutics targeting neutrophils, there is a need to better understand the mechanisms involved in the regulation of neutrophil numbers and their effector functions, which can be either harmful/beneficial in a context-dependent manner. In this review, we will discuss the basic biology of neutrophil production and how risk factors for cardiovascular inflammation influence neutrophil production and function. This is of critical importance as several therapeutic strategies for CVD seems to at least partly intersect with the granulopoiesis pathway. We also provide an overview of the contribution of neutrophils to various stages of atherosclerosis and its clinical manifestations including ACS and HF. Finally, we provide an update on the emerging strategies to bridle the harmful aspects of neutrophil activity.
2. Neutrophils are indispensable for a healthy response to infection and inflammation
Neutrophils are an integral part of the innate immune system in vertebrate life as they are the first responders in our defence against invading pathogenic microorganisms.5 They constitute nearly 70% of all white blood cells (WBCs) and owing to their short lifespan, they need to be constantly replenished to maintain a steady-state number. In response to local infection or injury, neutrophils rapidly adhere to activated endothelium, transmigrate into tissues, phagocytize pathogens, deploy anti-microbial proteins, produce reactive oxygen species (ROS), and neutrophil extracellular traps to eliminate microbes as quickly and efficiently as possible.6 Under certain sterile inflammatory conditions, such as ischaemic damage induced by myocardial infarction (MI), pro-inflammatory cues accelerate neutrophil activation and production resulting in tissue damage.7 To avoid the harmful effects on the host, neutrophil number and activation must be tightly regulated, It is therefore imperative that a fine balance between the pathways that regulate neutrophil production (granulopoiesis) and their elimination are in constant homeostasis. Neutrophil numbers in the circulation are regulated at various levels including the haematopoietic stem and progenitor cells (HSPC) via self-renewal, expansion, and differentiation in the bone marrow (BM), egress from BM, vascular demargination, transmigration into tissues, ageing, and rapid clearance by efferocytic macrophages.8 We discuss here, how neutrophil production is regulated during steady state and dysregulated during sterile inflammation in the context of CVD.
3. Production of neutrophils (granulopoiesis)
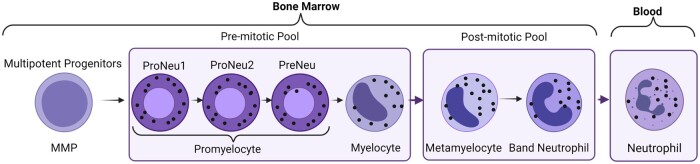
Granulopoiesis is a well-orchestrated de novo production of neutrophils from the haematopoietic stem cells (HSCs) within the haematopoietic cords of BM. The classical textbook scheme of neutrophil generation from HSCs involves a tree-like model with a series of stepwise differentiation steps from HSCs to neutrophils.9 This has now been replaced with a newer model, where HSCs give rise to lineage restricted progenitors without going through an ordered series of progressively lineage restricted multipotent progenitors (MPP).9 Nevertheless, MPP population arising from short-term (ST)-HSCs has further been subdivided into MPP1, MPP2, MPP3, and MPP4 populations based on stepwise gain of CD34, CD48, and CD135 as well as loss of CD150 expression.8 In mice, the MPP3 subset is biased towards neutrophils compared to the other MPPs.8 Hereafter, the neutrophil differentiation tree is divided into the pre-mitotic and post-mitotic pools (Figure 1).
Figure 1.
Production of neutrophils: neutrophil promyelocytes (subdivided into ProNeu1, ProNeu2, and PreNeu) are the first neutrophil committed progenitors in the neutrophil mitotic pool. Promyelocytes can be recognized by their round nucleus, relatively dark cytoplasm, and its ability to divide. Promyelocytes proliferate or differentiate into myelocyte, which are also the last cells that can divide. In the post-mitotic phase of neutrophil development, the non-dividing myelocytes mature into metamyelocytes and then band neutrophils. Metamyelocytes are characterized by the appearance of a bent kidney-shaped nucleus and clear cytoplasm, while banded cells have a horseshoe-shaped nucleus before their maturation into segmented neutrophils with lobular nuclei.
Neutrophil promyelocytes are the first neutrophil committed progenitors in the ‘mitotic neutrophil pool’ in humans.10 Promyelocytes can be recognized by their round nucleus, relatively dark cytoplasm, and their ability to divide. Promyelocytes proliferate or differentiate into the next stage, the myelocyte, which are the last cells in the neutrophil differentiation tree, which can divide.10 Human promyelocytes and myelocytes are not homogenous in their ability to divide. Under homeostatic conditions, a proportion of these cells (50%) divide slowly but can provide a pool of rapidly mobilizable neutrophils under emergency conditions.10 Recent studies using single-cell RNA sequencing and mass cytometry by time of flight have also identified novel intermediates connecting GMP to functionally mature neutrophils.10,11 Pro-neutrophils (ProNeu1) have been identified as the earliest unipotent progenitor population that gives rise to the very early immature neutrophil progenitor ProNeu2 and PreNeu, which further give rise to myelocytes.11–13
In the post-mitotic phase of neutrophil development, the non-dividing myelocytes mature into metamyelocytes and then to band neutrophils. Metamyelocytes are characterized by the appearance of a bent kidney-shaped nucleus and clear cytoplasm, while banded cells have a horseshoe-shaped nucleus before their maturation into segmented neutrophils with lobular nuclei. Metamyelocytes and band neutrophils are not found in the blood under homeostatic conditions but can be found in circulation during periods of acute inflammation, referred to as a left shift in neutrophil population.10
Neutrophils produced during steady state undergo terminal differentiation in the BM and the overall post-mitotic transit time in humans is estimated to be around 4–6 days with a daily turnover of 5–108 cells per day.10 In humans, the BM neutrophil pool is 6–8 times larger than the peripheral blood pool and serves as a rapidly mobilizable source for release during inflammatory stress.10 Neutrophils can also undergo terminal differentiation outside the BM. In fact, early unipotent neutrophil progenitors can be found outside the BM both in mice and humans.14 A comparison of BM and blood neutrophil transcriptome and epigenome suggest that neutrophils may mature outside BM.15 In fact, Hidalgo et al.10 suggest that mature neutrophils are in full equilibrium between blood, BM, and other tissue sites.
In a steady state, granulopoiesis is stimulated by granulocyte colony-stimulating factor (G-CSF), a growth factor that is mainly produced by immune cells, fibroblast, endothelial cells, and BM stromal cells.16 G-CSF production is down-regulated when the number of neutrophils increases and is up-regulated with neutrophil apoptosis in the BM. G-CSF has a wide range of effects from committing progenitor cells to myeloid lineage (via enhanced expression of PU.1 and C/EBPβ) to proliferation of granulocyte progenitors, reduction of transit time through the granulocytic compartment, and release of mature neutrophils from the BM via the suppression of CXC-chemokine receptor 4 (CXCR4)-stromal derived factor axis.17 G-CSF can also act as a negative regulator of neutrophil mobilization by acting as a neutrophil mobilizer at the relatively late stage of acute inflammation, but may also prevent exaggerated neutrophil mobilization during early-phase infection and inflammation.18 Emergency granulopoiesis can also occur in a G-CSF-independent manner in response to certain pathogens, but neutrophil effector function could be impaired due to the lack of G-CSF production.19 For example, the mice lacking G-CSF (Csf3−/−) have profound neutrophilia following Candida albicans infection, yet these animals remain more susceptible to candidiasis compared to wild type (WT) infected mice.19 Different cytokines can also induce the production of G-CSF or act in synergy with G-CSF.20 Granulopoietic factors, such as GM-CSF, interleukin(IL)-6, and IL-3 that are often increased during acute infections are also known to regulate emergency granulopoiesis.21 However, knockout out of either G-CSF, G-CSFR, GM-CSF and IL-6, or G-CSF and GM-CSF all demonstrated normal emergency granulopoiesis in response to sterile inflammatory stimuli suggesting that factors regulating granulopoiesis during sterile inflammation are likely different from the ones that regulate emergency granulopoiesis during acute infections.22
Following the release of mature neutrophils from the BM, they are distributed almost equally between two compartments, circulating and marginal.23 In a steady state, these two pools remain in dynamic equilibrium and are indistinguishable from each other. In humans, the marginated pool (i.e. adherent to endothelium) is present mainly in liver, spleen, and BM vasculature, while in some species it may also be present in the lungs.10 Demargination of neutrophils causes rapid neutrophilia and occurs in conditions of stress, such as pain, anxiety, excess physical activity owing to a surge in catecholamines and/or glucocorticoids.23 The mechanisms that promote demargination are unclear but likely involves shedding of L-selectin (CD62L)24 on the surface of neutrophils by cysteine metalloproteinases, such as Adam17.25,26 Demarginated neutrophils are much more softer and pliable owing to cytoskeletal reorganization of cortical actin27 and this phenomenon may also induce a change in the shape of neutrophils from spherical to amoeba-like. The physiological factors that promote margination in a steady state are unclear but in inflammatory conditions, cytokines, such as TNF-α and IL-1β stiffen leucocytes28 and enhance their migration towards endothelium consistent with the characteristics of an acute inflammatory response. The distribution of neutrophils within the system also varies and depends on the maturation and activation status. For example, mature peripheral blood neutrophils localize mainly to the liver while the younger cells home back to the BM.29
4. Perturbations in neutrophil production
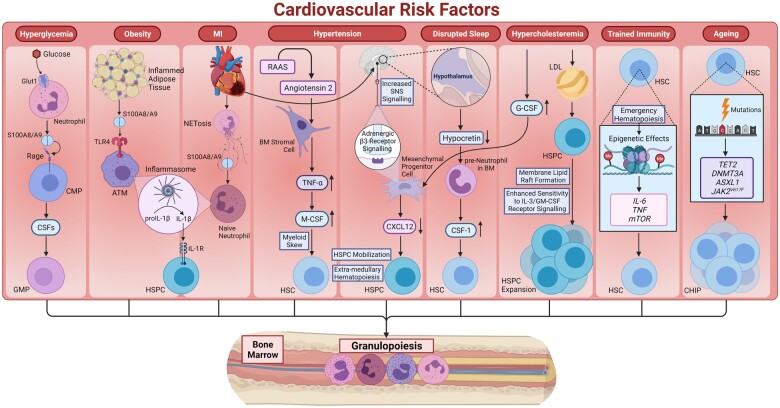
Neutrophilia is a hallmark of sterile inflammation. Several CVD risk factors, such as dyslipidaemia, diabetes, obesity, rheumatoid arthritis (RA), sleep deprivation, chronic variable stress, ageing, etc. have immense effects on myeloid-biased HSPC proliferation (Figure 2). In the following sections, we discuss the impact of these CVD risk factors and the underlying mechanisms that drive myelopoiesis, which is invariably linked to production of neutrophils.
Figure 2.
Perturbations in neutrophil production: several CVD risk factors can result in neutrophilia due to myeloid-biased HSPC proliferation. Hyperglycaemia can drive myelopoiesis by stimulating S100A8/A9 release from neutrophils, S100A8/A9 then binds to RAGE receptor on CMPs stimulating the production of CSFs. These CSFs can stimulate the excessive production of monocytes and neutrophils by GMPs. Excessive inflammation in adipose tissues also results in the release of S100A8/A9 and activation of IL-1β secretion by ATMs. This IL-1β can then stimulate myelopoiesis in the BM. MI in addition to stimulating IL-1β release from neutrophils can also lead to enhanced SNS signalling, which can drive HSPC mobilization and extramedullary proliferation. Hypertension is also associated with increased sympathetic activity leading to increased HSC mobilization. Angiotensin II produced by the RAAS system can cause increased production of TNF-α by BM stromal cells, which in turn increases M-CSF production resulting in myeloid skewing of HSPC differentiation. Disrupted sleep also reduces the level of hypocretin produced by hypothalamus, leading to increased production of CSF-1 by pre-neutrophils in the BM. CSF-1 then act on HSCs to increase the production of neutrophils. Hypercholesterolaemia can stimulate expansion and proliferation of HSPCs due to enhanced IL-3/GM-CSF receptor signaling resulting from greater lipid raft formation in HSPCs cell membrane. Hypercholesterolaemia is also linked to increased G-CSF levels, which can lead to decreased production of CXCL12 by mesenchymal progenitor cells causing greater HSPC mobilization and EMH. Inflammation-induced emergency haematopoiesis can result in long-term epigenetic effects on HSCs to generate higher number of monocytes possessing increased reactivity on second exposure. Ageing can also lead to accumulation of mutations in multiple genes (TET2, DNMT3A, ASXL1, and JAK2V617F), which confers a slight proliferative advantage to mutant stem cells. These mutations affect stem cell quiescence to promote HSPC expansion and introduce a bias in lineage fate selection, which can predispose to haematopoietic malignancies and CVD.
4.1 Dyslipidaemia, defective cholesterol handling, and granulopoiesis
Hypercholesterolaemia and defective cholesterol efflux pathways induce myelopoiesis both in the BM and spleen resulting in increased number of circulating monocytes and neutrophils. This elevated number of monocytes and neutrophils and the subsequent unstable atherosclerotic plaques are a direct consequence of increased accumulation of cholesterol in HSPCs in the BM and spleen.30 The ATP-binding cassette transporters, ABCA1 and ABCG1 play an important role in mediating cellular cholesterol efflux to apoA1 and HDL, respectively. The absence of or any defect in these efflux transporters in HSPCs accelerates atherosclerosis particularly when associated with hypercholesterolaemia.31 Greater accumulation of cholesterol in HSPCs promotes membrane lipid raft formation and enhances their sensitivity to IL-3/GM-CSF receptor signalling, which in turn stimulates expansion and proliferation of HSPCs.32 Various experimental strategies aimed at increasing cholesterol efflux from HSPCs, such as liver-X-receptor (LXR) agonists that up-regulate ABCA1 and ABCG1, overexpression of apoA1 and HDL all suppress myelopoiesis and neutrophil production.32,33 Dysregulated cholesterol efflux in BM osteoblastic and endothelial niche cells also stimulate myelopoiesis.33 Hypercholesterolaemia is also linked to increased G-CSF levels, which can lead to greater HSPC mobilization and extramedullary haematopoiesis (EMH).33 Hypercholesterolaemia also induces bone-loss34 resulting in remodelling of endosteal niche and greater HSPC mobilization and extramedullary myelopoiesis.
Patients with RA have a two–three-fold increased risk of developing atherosclerotic CVDs. Unlike other CVDs, RA patients do not display traditional CV risk factors, such as dyslipidaemia35 yet present prominent monocytosis and neutrophilia.36 Thus, identifying CVD risk in these patients remains challenging. Recent studies have identified the down-regulated expression of cholesterol efflux genes (Apoe, Abca1, and Abcg1) in HSPCs as the causative factor for enhanced and sustained myelopoiesis.37 Consequently, these alterations in cholesterol metabolism impair atherosclerotic lesion regression or alter lesion progression in pre-clinical models of atherosclerosis.37
4.2 Diabetes and granulopoiesis
Diabetes, a major risk factor for CVD, is also associated with increased number of circulating monocytes and neutrophils. Neutrophils exposed to hyperglycaemia associated with type I diabetes (T1D) undergo glycolytic stress and release excess amounts of alarmins, such as S100A8 and S100A9.38 These proteins preferentially form a hetero-oligomeric complex and then interact with the receptor for advanced glycation end products (RAGE) on common myeloid progenitors (CMP) in the BM to stimulate the production and release of colony-stimulating factors (CSFs) (M-CSF and GM-CSF). These CSFs in turn interact with their receptors on GMPs to stimulate the production of monocytes and neutrophils.38,39 Various strategies ranging from the reversal of hyperglycaemia, disruption of S100A8/A9-RAGE signalling axis and blockade of GLUT1, the main glucose transporter in neutrophils, all reduce glucose-driven myelopoiesis and atherosclerosis.38,39 Type 2 diabetes (T2D) is also associated with increased number of circulating monocytes and neutrophils.40 However, unlike in T1D, myelopoiesis in T2D is not overtly glucose-dependent but rather obesity-dependent. Excess inflammation in the adipose tissue, most likely due to adipocyte necrosis results in the release of S100A8/A9, which in turn interact with the toll-like-receptor (TLR4) on adipose tissue macrophages (ATMs). This interaction leads to induction and activation of the NLRP3 inflammasome and subsequent release of IL-1β, which in turn travel to the BM to stimulate myelopoiesis. IL-1β produced in response to hyperglycaemia and hypercholesterolaemia can cause myeloid biasing of HSCs through activation of a PU.1-dependent gene programme.41 Inflammasome expression in HSCs is necessary for myeloid lineage specification via caspase-1-dependent cleavage of GATA142 and hypercholesterolaemia can mediate long-lasting NLRP3 inflammasome-mediated reprogramming of myeloid cell progenitors to favour increased production of monocytes.43 The culmination of these events is a vicious feed-forward loop that provides adipose tissue with a constant supply of monocytes and neutrophils resulting in adipose tissue inflammation and insulin resistance.44,45 The recent COVID19 pandemic has also highlighted the dysregulation of this axis in obesity and diabetes, linking increased S100A8/A9 expression in WBCs to poor outcome in COVID19 infection by increasing inflammation, the pro-coagulant state, and pulmonary damage. This observation may contribute to the particularly poor outcome of COVID19 in individuals with a history of obesity, diabetes, or CVD, conditions where S100A8/A9 levels are thought to be already increased.46
4.3 Sleep deprivation and granulopoiesis
Sleep deprivation, another risk factor for CVD is also associated with dysregulated myelopoiesis.47 Disrupted sleep reduces the level of hypocretin produced by hypothalamus, leading to unfettered production of CSF-1 by pre-neutrophils in the BM. CSF-1 then act on HSCs to increase the production of neutrophils and monocytes, promoting atherosclerosis.48 These findings likely apply at least partly to the association between sleep apnoea and increased neutrophil counts.49
4.4 MI, stroke, variable stress, hypertension and granulopoiesis
MI, stroke, and chronic mild stress, all favour atherosclerotic plaque progression due to increased number of circulating monocytes and neutrophils.50 These conditions lead to enhanced sympathetic nervous system (SNS) signalling, a driver of HSPC mobilization and extramedullary proliferation.50 Increased sympathetic signalling after MI causes decreased CXCL12 production in perivascular niche due to activation of adrenergic β3 receptors on nestin+ mesenchymal stromal cells.51 Ischaemic stroke activates HSCs via increased β3-adrenergic signalling in HSCs, as well as changes in the BM niche, leading to a myeloid bias in haematopoiesis and higher BM output of inflammatory Ly6Chigh monocytes and neutrophils.52 Abdominal aortic aneurysm development is also characterized by enhanced myelopoiesis in an IL-27 dependent manner.53 Hypertension is also associated with elevated WBC count and disturbances in haematopoiesis.54 Increased sympathetic activity during hypertension leads to increased HSC mobilization that in turn promote CVD.55 Renin–angiotensin–aldosterone system, which drives hypertension, also has a role in regulation of haematopoietic system. Angiotensin II causes increased production of TNF-α by BM stromal cells, which increases M-CSF production resulting in myeloid skewing of HSPC differentiation.56 Neutrophils in the BM also express adrenergic β receptors and hyperactivation of SNS during hypertension stimulates neutrophils to produce proteases like MMPs that can cleave CXCR4, leading to an increase in circulating leucocytes.55 Haematopoiesis can additionally switch to an EMH mode and ramp up neutrophil production to meet the increased demand of neutrophils during stressful conditions. HSPCs also circulate in the bloodstream for immunosurveillance and can seed extramedullary tissues (spleen and liver) under acute and chronic stress conditions (i.e. infection, MI, or cancer).57
4.5 Ageing and granulopoiesis
Ageing is also associated with decline in the stemness of HSCs and a bias towards the myeloid lineage. Low levels of chronic inflammation, reduced telomere length, impaired autophagic removal of defective mitochondria leading to mitochondrial stress, altered autophagy resulting in dysregulated proteostasis, replicative stress can all contribute to stem cell ageing.58 Ageing can cause accumulation of mutations in multiple genes including methylcytosine dioxygenase 2 (TET2), DNMT3A, ASXL1, and the gain of function mutation in JAK2V617F leading to what is known as clonal haematopoiesis of indeterminate potential (CHIP).59 These mutations confer a slight proliferative advantage to mutant stem cells allowing them to outcompete non-mutant stem cells in colonizing stem cell niches. They promote HSPC expansion and introduce a bias in lineage fate selection, predisposing to haematopoietic malignancies and various CVDs.60–63 This was validated when partial BM reconstitution with TET2-deficient HSPC caused a marked increase in atherosclerotic plaque size, increased NLRP3 inflammasome activation in macrophages60 and caused greater cardiac dysfunction in murine models of HF.61 Similarly haematopoietic Jak2V617F expression promotes early lesion formation, increased neutrophil infiltration in early lesions, increased erythrophagocytosis and defective efferocytosis leading to greater plaque instability.62 Mice with conditional knock-in Jak2V617F also have an increased propensity for NET formation and thrombosis.63
Ageing is also associated with impaired clearance of apoptotic neutrophils by efferocytic macrophages in the peripheral organs, such as the lungs BM and spleen. IL-23 production was suppressed in efferocytic macrophages phagocytosing apoptotic neutrophils, leading to a feedback loop controlling G-CSF and granulopoiesis, regulating overall neutrophil numbers in blood.64,65 Thus, impaired clearance of apoptotic neutrophil by efferocytic macrophages maybe a major contributor of perturbed neutrophil homeostasis observed with age or chronic inflammation. Moreover, inefficient clearance capacity of macrophages is observed in chronic inflammatory diseases, such as atherosclerosis.8 In addition, macrophages with metabolic perturbations due to myeloid-specific deficiency of Glut1, cholesterol efflux transporters, Ucp2, or mitochondrial complex III are also inefficient in efferocytosis.8
4.6 Gut microbiome and granulopoiesis
The gut microbiome plays a crucial role in regulating steady-state granulopoiesis. In fact, antibiotic usage is generally believed to be associated with suppressed myelopoiesis in the BM.66 Microbial components including ligands for TLRs can induce IL-17 production from intestinal innate lymphoid cells resulting in the production of G-CSF.66 Neutrophil recruitment to the mucosal system is also involved in feedback suppression of G-CSF production.66 NOD1 ligands secreted by microbiota can also be sensed by BM stromal cells to regulate haematopoiesis.66 In addition, the microbiome also links nutrition with inflammation. Hypercholesterolaemia, hyperlipidaemia, and high salt consumption can modify the gut microbiome composition, which in turn influences the development of cardio-metabolic diseases.67 Long-term high-fat diet (HFD) can alter gut microbiota and lead to changes in BM niche resulting in myeloid biasing of long-term Lin−Sca-1+c-Kit+ (LSK) stem cells.68 Transplantation of pro-inflammatory faecal microbiota can amplify blood neutrophil counts and accelerate atherosclerosis development.69 Similarly, neutrophilia associated with altered gut microbiota in aged mice leads to sustained inflammation and HF after MI.70 Even in human studies, faecal microbiota transplantations are gaining traction as a potential treatment modality to correct a dysregulated immune response in a range of metabolic and haematologic conditions.71 Antibiotic-induced alterations in the intestinal flora reduce ischaemic brain injury in mice, gut microbiota depletion with antibiotic treatment reduced migration of intestinal IL-17-producing γδ T cells from gut to the brain after stroke; thereby improving outcomes after ischaemic stroke.72 Finally, microbiota also regulate neutrophil ageing leading to a pro-inflammatory neutrophil phenotype driving vascular damage, and depletion of microbiota significantly reduces organ damage in sickle cell disease, septic shock, and MI.66,73,74 However, a neutrophil intrinsic molecular clock regulating neutrophil ageing has also been proposed, and the precise mechanism of action of the microbiome acting on the neutrophil and its BM progenitors remains to be revealed.73 Taken together, these studies point towards a role for microbiota in neutrophil activation state and neutrophilia, but causal evidence for microbiota in chronic cardiovascular inflammation is still lacking.67 Given the novelty of this field and the relative lack of human studies; the precise microbial species and mechanisms driving this association remain to be disentangled. This an important notion, as perturbations in the microbiome likely represent a shift in the balance of species that have either pro- or anti-inflammatory effects on the neutrophil. Isolation and supplementation of beneficial species (probiotics) or supplementation of anti-inflammatory microbial metabolites (postbiotics) are potential clinical applications to reduce CVD derived from a deeper insight into the role of the microbiome in human disease.71
4.7 Trained immunity and granulopoiesis
Innate immune cells can mount a non-specific memory response. This phenomenon akin to immunologic memory of adaptive immunity is called ‘trained immunity’. Inflammation-induced emergency haematopoiesis can result in long-term epigenetic effects on HSCs, generating higher numbers of monocytes possessing increased reactivity to second exposure. Epigenetic analysis of HSCs after inflammatory exposure has identified histone modifications in genes encoding inflammatory cytokines, such as IL-6 and TNF-α along with the genes coding for the mTOR pathway.75,76 Trained immunity also occurs during sterile inflammation. Western diet feeding of Ldlr −/− mice for 4 weeks results in systemic inflammation, that return to steady-state levels when switched back to normal chow. In spite of this, BM and splenic myeloid cells remain hyper-activated as measured by inflammatory cytokine production upon TLR stimulation.43 Similarly, systemic response to ischaemic injury caused by MI or stroke aggravates chronic atherosclerosis by increasing monocyte production and recruitment to plaques.51 Cytokines, such as IFN-γ, IFN-α, and IL-1β produced during inflammation, could activate HSPCs.41,42,77,78 Unexpectedly an MI-induced BM ‘memory’ dampens the organism’s reaction to subsequent events.79 Recurrent MI caused reduced emergency haematopoiesis and less leucocytosis than the first MI. The haematopoietic response to lipopolysaccharide was also mitigated after a previous MI.79
5. Neutrophils and atherogenesis
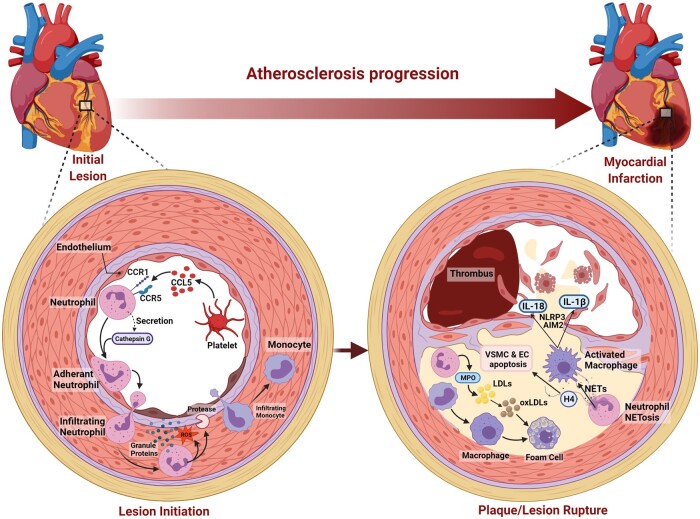
Atherosclerosis is the most common cause of MI and stroke making it a leading cause of death and disability in the developing world.80 Atherosclerosis is characterized by arterial wall damage caused by increased accumulation of apolipoprotein B-containing lipoproteins in the intimal space of arteries and a maladapted inflammatory response. Arterial endothelial cell activation leads to myeloid cell infiltration into the arterial intima contributing to atherosclerotic plaque growth and instability, leading to plaque rupture and cardiovascular complications (Figure 3).67 In recent years, neutrophils have received enormous attention for their role in sterile inflammation associated with CVD due to the discovery of NETosis and NLRP3 inflammasome signalling in neutrophils. The number of circulating neutrophils correlates positively with the size of developing lesions. Furthermore, depletion of Ly6G+ neutrophils in Apoe−/− mice protect against the development of atherosclerosis.81 Atherosclerosis develops at sites of disturbed flow and increased shear stress, which leads to endothelium activation resulting in increased permeability and leucocyte adhesion.82 Neutrophils play a major role in the initiation of atherosclerosis by dysregulating vascular endothelial cells and triggering leucocyte arrest, thus setting the stage for atherosclerosis.83 Neutrophil granule proteins in addition to directly activating the endothelial cells also degrade the underlying extracellular matrix leading to further adhesion of monocytes, vascular hyper-permeability, and transfer of LDL particles.84 During the initial phase of atherogenesis, chemotactic molecules guide the recruitment of neutrophils to atherosclerosis susceptible areas. Cathepsin G (CatG), an anti-microbial polypeptide deposited by neutrophils on arterial endothelial cells after HFD feeding activates leucocytes to firmly adhere via integrin clustering. CatG neutralization abrogates arterial leucocyte adhesion without affecting myeloid cell adhesion in the micro-circulation.85 Differential expression of cell adhesion molecules on large arteries compared to micro-circulation may explain differential presentation of CatG and may account for specific control of myeloid cell adhesion. In mice, activated platelets deliver CC-chemokine ligand 5 (CCL5), the ligand for CCR1 and CCR5 on the endothelium of arteries, and promote CatG secretion from neutrophils promoting their accumulation.81,85 Chemokines also regulate the recruitment of neutrophils to arteries and peripheral venules. CC-chemokine receptor 1 (CCR1), CCR2, CCR5, and CXCR2 govern neutrophil infiltration in large arteries, while only CCR2 and CXCR2 do so in small vessels of mice.81 Neutrophils may infiltrate into the early atherosclerotic lesions from the arterial lumen86 or through the venules in the advanced stages of atherosclerosis.87 Neo-vasculature formed into the atherosclerotic lesions may also be a point of entry for neutrophils.88 In certain inflammatory settings, neutrophils can drive angiogenesis,89 suggesting the possibility that neutrophils can drive atherosclerotic plaque angiogenesis and promote its own recruitment or exit. Cathelicidins secreted by neutrophils along the lumen of injured arteries can activate circulating endothelial progenitor cells, which then contribute to re-endothelialization directly or by release of angiogenic growth factors.90 Neutrophils can undergo migration in a luminal to abluminal direction, this was observed in inflammation following ischaemia-reperfusion injury and is characterized by reduced expression of junctional adhesion molecule C from EC junctions.91 In a mouse hepatic injury model, infiltrating neutrophil neither die at the injury site nor are phagocytosed. Instead, these neutrophils re-enter the vasculature, travel to the lungs and up-regulate CXCR4 (C-X-C motif chemokine receptor 4) before ending up in the BM, where they undergo apoptosis.92
Figure 3.
Neutrophils and atherogenesis: neutrophils play a major role in initiation of atherosclerosis by dysregulating vascular endothelial cells and triggering leucocyte arrest. Activated platelets deliver CCL5, on the endothelium of arteries, CCL5 by itself or via promoting CatG secretion of from neutrophils promote leucocyte adherence on endothelium. Neutrophil granule proteins also degrade the underlying extracellular matrix leading to vascular hyper-permeability, further adhesion and entry of monocytes, and transfer of LDL particles. Myeloperoxidase from neutrophils also oxidize LDL to accelerate foam cell formation. NET formation by neutrophils can also drive atherosclerosis through the activation of macrophages leading to production of IL-1β. Histone H4 secreted during NETosis can lead to VSMC cell death and thinning of fibrous cap and plaque rupture. Activated macrophages or neutrophils can cause endothelial cell death by apoptosis or produce proteases, which disrupt endothelial cell adhesion to the vessel wall resulting in endothelial denudation and thrombus formation. Taken together, neutrophils play a major role in advanced or late-stage atherosclerosis where they destabilize and trigger plaque erosion or rupture.
Recent studies show that 40% of all protein-coding genes show circadian rhythms in their transcription93 and circadian rhythms also regulate the recruitment of neutrophils and monocytes into atherosclerotic lesions, which peaks during the morning hours. The overall rhythmicity of leucocyte adhesion to inflammatory sites seems to the dependent on Bmal1-regulated leucocyte clock.94 In spite of this, there seems to be a 24 hr rhythm shift in leucocyte recruitment in arteries and veins of mouse macro and microvasculature.95 Leucocyte adhesion to the arteries is highest in the morning, while it peaks at night for the veins.94 De Juan et al.94 show that vessel-specific oscillatory patterns govern the release of pro-migratory molecules. Endothelial cell-specific Bmal1 depletion disrupted time-of-day-dependent leucocyte adhesion in veins but not in arteries. This difference in leucocyte adhesion was ablated when β2-adrenergic-receptor signalling in sympathetic nerves was disrupted. Thus, circadian clock regulation of sympathetic nerves in large arteries, but not of veins, leads to increased levels of endothelial adhesion molecules resulting in enhanced neutrophil recruitment.94 Andover et al.73 recently demonstrated that steady-state leucocyte trafficking into tissues is regulated by a molecular clock in both leucocytes and endothelial cells. Specifically, Bmal1 expression in different leucocyte subsets or ECs is linked to integrins and chemokine receptors on leucocytes and expression of ICAM-1 and VCAM-1 on vascular beds.
Neutrophils also regulate monocyte entry into atherosclerotic lesions and cause macrophage activation. Myeloperoxidase released from neutrophils promote oxidation of LDL and accelerate foam cell formation.96 Neutrophil granule proteins, heparin-binding protein, and human neutrophil peptides can also activate human and mouse macrophages towards a pro-inflammatory phenotype that in turn could promote atherosclerosis.97 Neutrophil granule components, can also be secreted by NETosis and pharmacological inhibition of NET formation has been shown to reduce atherosclerosis development.98 NETs can drive atherosclerosis through the activation of macrophages leading to production of IL-1β.99 Neutrophil serine proteases can also induce cleavage of pro-IL-1β into its mature form. Thus, neutrophils play a central role in atherosclerosis development.
6. Neutrophils and atherosclerotic plaque instability
Rupture prone atherosclerotic lesions are dominated by macrophages, have large necrotic cores and a fibrous cap composed of vascular smooth muscle cells (VSMCs) and collagen.100 Activated macrophages or neutrophils can cause endothelial cell death by apoptosis or produce proteases, which disrupt endothelial cell adhesion to the vessel wall resulting in endothelial denudation. This process known as endothelial erosion, exposes the underlying connective tissue matrix, allowing platelets to adhere at the site resulting in thrombus formation.100 Alternatively, thrombus formation may be a result of plaque disruption. The plaque cap tears away exposing the lipid core, resulting in thrombus formation from within the plaque, which then extends into the arterial lumen.100
Neutropenia and neutrophilia were shown to respectively increase or decrease atherosclerotic plaque stability in mice and neutrophil levels in the arterial intima positively correlate with signs of plaque instability in both humans and mice.1 Thin fibrous caps in human atheroma specimens also show higher neutrophil counts than stable lesions, indicating a role for neutrophils in plaque destabilization. Increased infiltration of neutrophils into advanced lesions has been shown to induce collagen degradation and necrosis in the lesions.101 Mechanistically, activated neutrophils in the atheroma undergo NETosis to form NETs. These NETs can stably present a variety of neutrophil granule proteins (MPO, NADPH oxidase, nitro oxidase synthase), which can reduce the stability of atherosclerotic plaques. Cationic histone H2a bound on NETs can also accelerate recruitment of negatively charged classical monocytes into atherosclerotic lesions.102 Histones presented on NETs can also induce tissue damage. Histone H4 originating from intimal NETs can form membrane pores resulting in smooth muscle cell death and vascular tissue damage leading to destabilization of plaques1. Additionally, NETs can also activate ‘absent in melanoma 2 (AIM2) inflammasome’ in macrophages103 to produce the proatherogenic cytokines IL-1β and IL-18 leading to unstable atherosclerotic lesion.103 JAK2V617F mutation, a common genetic variant that increases JAK-STAT signalling and imparts greater risk of premature MI and stroke104 and promote CHIP, can exacerbate atherosclerosis via AIM2 inflammasome.105 Deletion of the critical inflammasome components, such as caspase 1 and 11, Gasdermin D or Inhibition of IL-1β, all reduced necrotic core formation while increasing the stability of the plaques.105 Together, these studies suggest that neutrophils play a major role in advanced or late-stage atherosclerosis where they destabilize and trigger plaque erosion or rupture.
7. Neutrophils in acute myocardial infarction
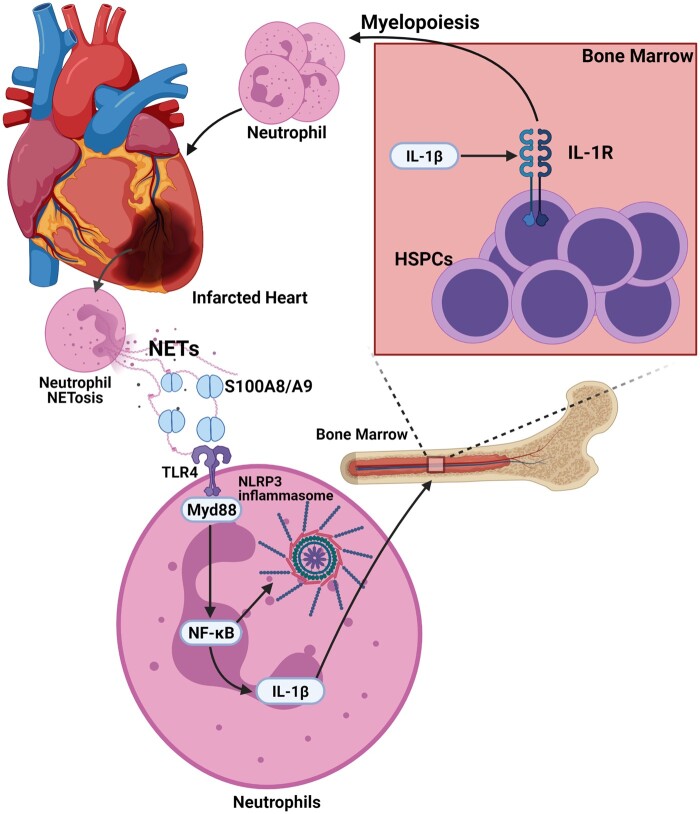
Acute myocardial infarction (AMI) is a major cause of HF and HF-related morbidity and mortality. Neutrophil count in the blood and the ratio of neutrophils to total leucocytes, particularly lymphocytes correlate well with the severity and complexity of CAD, large MI, recurrent ischaemic events, and mortality.106 Rupture of an atherosclerotic plaque in vital organs, such as heart and brain can lead to obstruction of blood circulation resulting in ischaemic/necrotic death of tissues that immediately triggers an acute inflammatory response spearheaded by neutrophils. Attracted by cellular debris including alarmins and inflammatory signals released by dead cells, neutrophils massively infiltrate the infarct area within hours.7 At the site of injury, activated neutrophils generate and release excess amounts of ROS, proteases, and in some instances the entire cytoplasm and nuclear material (NETs).107 In the context of sterile inflammation, this behaviour of neutrophils may result in an exacerbated injury to the tissue. For example, S100A8/A9 released by neutrophils during an acute MI can prime the NLRP3 inflammasome on naïve neutrophils to release IL-1β, which then stimulates granulopoiesis in the BM leading to increased accumulation of neutrophils in the injured heart, resulting in adverse remodelling and eventual HF (Figure 4).7,107
Figure 4.
Neutrophils during MI: following AMI, neutrophils accumulate at the site of injury and mount an acute inflammatory response. These neutrophils undergo NETosis to release S100A8/A9 into the infarct and prime the NLRP3 inflammasome on naïve neutrophils to release IL-1β. IL-1β then stimulates granulopoiesis in the BM leading to increased accumulation of neutrophils in the injured heart, resulting in adverse remodelling and eventual HF.
Neutrophil production in the BM is also time-of-day dependent as experimental MI induced at the beginning of the active phase resulted in higher cardiac neutrophil infiltration resulting in excessive inflammation and sub-optimal cardiac repair.108 Neutrophils are also major contributor to ischaemia/reperfusion injury, due to release of ROS and other inflammatory mediators resulting in cardiomyocyte apoptosis, especially at the ischaemic border zone.109 Release of MPO by infiltrating neutrophils results in oxidative stress, generation of cytotoxic aldehydes, and activation of extracellular matrix degrading enzymes and further leucocyte infiltration leading to maladaptive remodelling.109 Complete MPO depletion or inhibition has been found to mitigate inflammation and reduce left ventricular dilatation and dysfunction.110 Interference with neutrophil recruitment has also been shown to have beneficial effect after IR injury with reduced infract size, reduced left ventricular fibrosis, and greater preservation of cardiac function and prevention of neutrophil extracellular trap formation.4
Despite the many studies showing a beneficial role for neutrophil targeted approaches in experimental I/R injury, other studies failed to show any beneficial effects.4 For example, long-term depletion of neutrophils in experimental MI did not improve but rather impaired the resolution of inflammation resulting in adverse remodelling and decreased cardiac function.111 Although, experimental strategies aimed at reducing neutrophil activity have been shown to limit tissue injury, translating this into clinical practice has been elusive.4 Accumulating evidence suggests that neutrophils are not always detrimental and thus, cannot be conferred an exclusive ‘inflammation only’ function. Many recent studies have shown that neutrophils possess anti-inflammatory and reparative function in the context of bacterial infection and non-sterile injury.112 These observations have led us to hypothesize that there exists a threshold of neutrophil-induced inflammation that is necessary to kick start the reparative process and beyond this threshold, greater number of neutrophils and extended residency is detrimental. This neutrophil number threshold may also be differentially tolerated depending on the time of injury due to a cell intrinsic programme of neutrophil proteome disarming.112
8. Neutrophils and myocardial repair
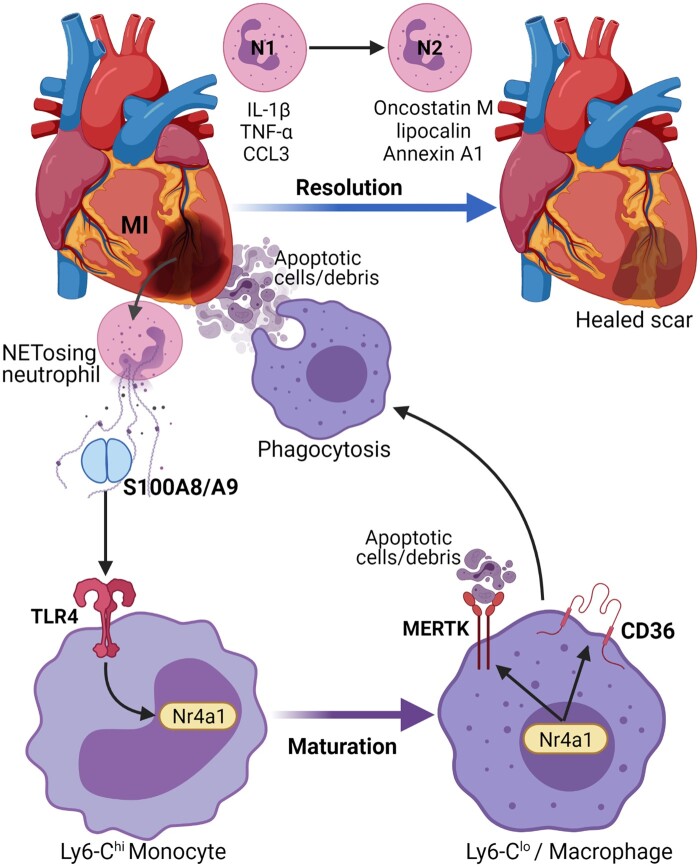
Beyond the established pro-inflammatory role of neutrophils during MI and I/R induced damage, neutrophils are also involved in the modulation of healing and remodelling response (Figure 5). After neutrophil infiltration, monocytes and monocyte-derived macrophages infiltrate the infarct area to remove dead cell debris and apoptotic neutrophils, activating reparative pathways necessary for proper healing.113 Macrophage phagocytosis of apoptotic neutrophils also activate an anti-inflammatory response marked by production of IL-10, transforming growth factor-β and pro-resolving lipid mediators and suppression of pro-inflammatory cytokines.113 Neutrophil-derived DAMPs, such as S100A8/A9 can facilitate conversion of inflammatory monocytes to reparative macrophages through induction of nuclear receptor subfamily 4 group A member 1 (Nr4a1) expression.114 Nr4a1 is also needed for up-regulation of Mertk, the phagocytosis receptor115 required for efficient clearance of debris and dead cells.116 The transcriptional profile of neutrophils in the heart also undergoes a temporal shift from pro-inflammatory N1 to pro-resolving N2 neutrophils. The N1–N2 neutrophils are categorized as Ly6G+CD206− and Ly6G+CD206+ cells, respectively. The N1 cells also express pro-inflammatory markers, such as IL-1β, TNF-α, CCL3 on Day1 while the N2 cells display anti-inflammatory markers on Days 5–7, which aid in the resolution of inflammation and clearance of apoptotic cardiomyocytes.117 Neutrophils and macrophages also produce oncostatin M, that can dedifferentiate cardiomyocytes to release regenerating islet-derived protein 3β, and promote reparative macrophage accumulation in the heart.118 A subset of pro-angiogenic neutrophils (CXCR4highVEGFR+CD49d+) controlling the regrowth of blood vessels has also been identified.89,92 Alternatively neutrophils may also drive angiogenesis via indirect mechanism by activating macrophages to a angiogenic phenotype.119 Annexin A1 produced by neutrophils can also polarize macrophages to a pro-angiogenic phenotype.119
Figure 5.
Neutrophils and myocardial repair: neutrophils are also involved in modulation of healing and remodelling response after an MI. Neutrophil-derived S100A8/A9 induce the expression of Nr4a1 in inflammatory monocytes and their conversion to reparative macrophages. This also leads to up-regulation of Mertk and CD36, the phagocytosis receptor required for efficient clearance of dead cells. The transcriptional profile of neutrophils also changes from a pro-inflammatory N1 profile expressing markers like IL-1β, TNF-α, and CCL3 on Day1 to an N2 anti-inflammatory profile aiding in the resolution of inflammation. In addition, neutrophils also express pro-reparative factors like oncostatin M, lipocalin, and Annexin A1. In summary, neutrophils are essential for jumpstarting the reparative responses after an ischaemic injury.
Due to the quintessential role of neutrophils in jumpstarting the reparative response, anti-neutrophil strategies aimed at reducing neutrophil influx to limit acute post-ischaemic tissue injury is likely to suppress the subsequent healing response. Previous studies have demonstrated that long-term depletion of neutrophil in MI induced by permanent left anterior descending coronary artery ligation resulted in a worsened cardiac function, increased fibrosis, and a progressive increase in biomarkers associated with HF.111 This was accompanied by reduced cardiac expression of myeloid-epithelial-reproductive tyrosine kinase (MerTK) on macrophages. Induction of MerTK expression on reparative macrophages was mediated by neutrophil-derived lipocalin.111 ST inhibition of neutrophils by the β1-adrenergic-receptor antagonist metoprolol has also been shown to reduce infarct size in AMI120 and early intravenous administration of metoprolol before reperfusion reduced infarct size and increased LVEF with no excess adverse events during the first 24 h after STEMI.121 Similar to the suppression of neutrophils, long-term inhibition of S100A8/A9 leads to deterioration of cardiac function over time114 while ST inhibition of S100A8/A9 mitigated both local and systemic inflammation, and as expected reduced myocardial damage after MI.122 S100A8/A9, may in fact, be the main effector molecules used by neutrophils to manage these contrasting inflammatory and reparative function of macrophages. These contrasting results from ST vs. long-term inhibition of S100A8/A9 illuminates the need to identify the right window of therapeutic opportunity to effectively suppress the inflammatory functions of neutrophils just enough to prevent excessive damage while still retaining their reparative functions. Pharmacological strategies aimed at selective inhibition of S100A8/A9, or the neutrophil machinery (e.g. Nlrp3 inflammasome) involved in the production of inflammatory mediators like IL-1β may represent a viable alternative as opposed to indiscriminate targeting of neutrophils by methods, such as neutrophil depletion and/or neutrophil recruitment.7,107 Further, screening of neutrophil proteome and/or lipidome post-MI for potential mediators of reparative processes (e.g. resolvents) are likely to aid in the development of novel approaches for therapeutic intervention.
9. Therapeutic targeting of neutrophils
The CANTOS and LoDoCo2 trials have targeted the IL-1β and IL6 pathways, which are upstream of granulopoiesis with clear cardiovascular benefit. At least in the setting of CANTOS trial, the benefits accrued seemed to have been offset by an increased risk of lethal infections that may have been attributed to decrease in neutrophil counts.123 These findings argue for a careful tuning of the neutrophil phenotype to reap optimal benefit from anti-inflammatory compounds. A better understanding of neutrophil effectors that are involved in the inflammatory and reparative functions would allow us to take a more targeted approach in developing effective therapies. This should allow us to exploit the inflammation-resolving function of neutrophils while restricting the collateral damage caused by an inflammatory overkill. Despite the failure of many previous attempts, many ongoing clinical trials targeting chemokines to inhibit neutrophil infiltration into inflamed tissues are in the pipeline, for example, using a small molecule inhibitor against CCR5124 and CXCR2.125 Neutrophil-derived alarmins, such as S100A8 and S100A9, have been shown to possess major roles in determining the nature and magnitude of inflammatory response post-MI and during the resolution of inflammation.114 Developing novel neutrophil-targeted therapeutics and compounds targeting S100A8/A9 may represent a departure from status quo in managing ischaemic CVD. Previous studies have shown that blockade of S100A8/A9 interaction with its receptors, TLR4 and RAGE, reduces inflammation and disease progression in a number of inflammatory models and could be repurposed to target inflammation in the heart post-MI.126 Inhibition of IL-1β release from neutrophils by targeting NLRP3 inflammasome has been shown to maintain atherosclerosis at a sub-clinical level.127 These inhibitors also demonstrated damage mitigation to the heart muscle post-MI suggesting a promising potential for treatment of HF.128
Preventing NET- driven inflammation is another promising target in controlling cardiovascular inflammation. NET chromatin degradation with systemic DNase I treatment has been shown to prevent cardiovascular inflammation and thrombosis.129 NET chromatin also can activate AIM2 inflammasome in macrophages, leading to IL-1β and IL-18 production in atherosclerotic lesions and inhibition of AIM2 inflammasome in mice increased lesion stability103 and may represent a target downstream of NET release. Therapeutic neutralization of histones bound on NETs can also be used to reduce monocyte infiltration into atherosclerotic lesions and prevent SMCs death to stabilizes atherosclerotic lesions.1,102 Neutrophil interaction with platelets is important for NET release and disruption of platelet-neutrophil communication might also limit NET release.130 Histone citrullination mediated by the enzyme PAD4131 can be inhibited to prevent NET release and reduce vascular inflammation in mouse models of atherosclerosis.131 Interventions to reduce S100A8/A9 secretion by inhibiting NETosis may also have suppressive effect on inflammasome activation and subsequent secretion of IL-β.107
10. Conclusion
Neutrophils in addition to being a central player in an inflammatory response during infections and tissue injury are also essential for homeostatic functions. Our understanding of neutrophil biology has been refined with new findings on the regulatory mechanisms that govern their production, maturation, and demise. There is also a newfound appreciation for the flexible, responsive nature of neutrophil transcriptional machinery, extended lifespan and multiple neutrophil sub-types in health and disease. The future challenges now depend on our ability to translate the newly found knowledge about neutrophil biology into therapeutics by striking a balance between the pro- and anti-inflammatory properties of neutrophils in CVD.
Funding
P.R.N. is supported by grants from the National Institute of Health (NIH) (R01HL1379, R21AG063197). N.M.J.H. is supported by a Diabetes Fonds - Diabetes Onderzoek Nederland (DFN-DON) grant 2020 (2020.10.002).
Contributor Information
Gopalkrishna Sreejit, Department of Surgery, The Ohio State University Wexner Medical Center, 473 W, 12th Ave, DHLRI 611A, Columbus, OH 43210, USA.
Jillian Johnson, Department of Surgery, The Ohio State University Wexner Medical Center, 473 W, 12th Ave, DHLRI 611A, Columbus, OH 43210, USA.
Robert M Jaggers, Department of Surgery, The Ohio State University Wexner Medical Center, 473 W, 12th Ave, DHLRI 611A, Columbus, OH 43210, USA.
Albert Dahdah, Department of Surgery, The Ohio State University Wexner Medical Center, 473 W, 12th Ave, DHLRI 611A, Columbus, OH 43210, USA.
Andrew J Murphy, Division of Immunometabolism, Baker Heart and Diabetes Institute, 75 Commercial Road, Melbourne, VIC 3004, Australia.
Nordin M J Hanssen, Amsterdam Diabetes Centrum, Amsterdam University Medical Centre, Location Academic Medical Centre Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands.
Prabhakara R Nagareddy, Department of Surgery, The Ohio State University Wexner Medical Center, 473 W, 12th Ave, DHLRI 611A, Columbus, OH 43210, USA.
References
- 1. Silvestre-Roig C, Braster Q, Wichapong K, Lee EY, Teulon JM, Berrebeh N, Winter J, Adrover JM, Santos GS, Froese A, Lemnitzer P, Ortega-Gomez A, Chevre R, Marschner J, Schumski A, Winter C, Perez-Olivares L, Pan C, Paulin N, Schoufour T, Hartwig H, Gonzalez-Ramos S, Kamp F, Megens RTA, Mowen KA, Gunzer M, Maegdefessel L, Hackeng T, Lutgens E, Daemen M, von Blume J, Anders HJ, Nikolaev VO, Pellequer JL, Weber C, Hidalgo A, Nicolaes GAF, Wong GCL, Soehnlein O. Externalized histone H4 orchestrates chronic inflammation by inducing lytic cell death. Nature 2019;569:236–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guasti L, Dentali F, Castiglioni L, Maroni L, Marino F, Squizzato A, Ageno W, Gianni M, Gaudio G, Grandi AM, Cosentino M, Venco A. Neutrophils and clinical outcomes in patients with acute coronary syndromes and/or cardiac revascularisation. A systematic review on more than 34,000 subjects. Thromb Haemost 2011;106:591–599. [DOI] [PubMed] [Google Scholar]
- 3. Chia S, Nagurney JT, Brown DF, Raffel OC, Bamberg F, Senatore F, Wackers FJ, Jang IK. Association of leukocyte and neutrophil counts with infarct size, left ventricular function and outcomes after percutaneous coronary intervention for ST-elevation myocardial infarction. Am J Cardiol 2009;103:333–337. [DOI] [PubMed] [Google Scholar]
- 4. Vinten-Johansen J. Involvement of neutrophils in the pathogenesis of lethal myocardial reperfusion injury. Cardiovasc Res 2004;61:481–497. [DOI] [PubMed] [Google Scholar]
- 5. Amulic B, Hayes G. Neutrophil extracellular traps. Curr Biol 2011;21:R297–R298. [DOI] [PubMed] [Google Scholar]
- 6. Ley K, Hoffman HM, Kubes P, Cassatella MA, Zychlinsky A, Hedrick CC, Catz SD. Neutrophils: new insights and open questions. Sci Immunol 2018;3:eaat4579. [DOI] [PubMed] [Google Scholar]
- 7. Sreejit G, Abdel-Latif A, Athmanathan B, Annabathula R, Dhyani A, Noothi SK, Quaife-Ryan GA, Al-Sharea A, Pernes G, Dragoljevic D, Lal H, Schroder K, Hanaoka BY, Raman C, Grant MB, Hudson JE, Smyth SS, Porrello ER, Murphy AJ, Nagareddy PR. Neutrophil-derived S100A8/A9 amplify granulopoiesis after myocardial infarction. Circulation 2020;141:1080–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yvan-Charvet L, Ng LG. Granulopoiesis and neutrophil homeostasis: a metabolic, daily balancing act. Trends Immunol 2019;40:598–612. [DOI] [PubMed] [Google Scholar]
- 9. Laurenti E, Gottgens B. From haematopoietic stem cells to complex differentiation landscapes. Nature 2018;553:418–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hidalgo A, Chilvers ER, Summers C, Koenderman L. The neutrophil life cycle. Trends Immunol 2019;40:584–597. [DOI] [PubMed] [Google Scholar]
- 11. Hedrick CC, Malanchi I. Neutrophils in cancer: heterogeneous and multifaceted. Nat Rev Immunol 2022;22:173–187. [DOI] [PubMed] [Google Scholar]
- 12. Evrard M, Kwok IWH, Chong SZ, Teng KWW, Becht E, Chen J, Sieow JL, Penny HL, Ching GC, Devi S, Adrover JM, Li JLY, Liong KH, Tan L, Poon Z, Foo S, Chua JW, Su IH, Balabanian K, Bachelerie F, Biswas SK, Larbi A, Hwang WYK, Madan V, Koeffler HP, Wong SC, Newell EW, Hidalgo A, Ginhoux F, Ng LG. Developmental analysis of bone marrow neutrophils reveals populations specialized in expansion, trafficking, and effector functions. Immunity 2018;48:364–379.e368. [DOI] [PubMed] [Google Scholar]
- 13. Kwok I, Becht E, Xia Y, Ng M, Teh YC, Tan L, Evrard M, Li JLY, Tran HTN, Tan Y, Liu D, Mishra A, Liong KH, Leong K, Zhang Y, Olsson A, Mantri CK, Shyamsunder P, Liu Z, Piot C, Dutertre CA, Cheng H, Bari S, Ang N, Biswas SK, Koeffler HP, Tey HL, Larbi A, Su IH, Lee B, St John A, Chan JKY, Hwang WYK, Chen J, Salomonis N, Chong SZ, Grimes HL, Liu B, Hidalgo A, Newell EW, Cheng T, Ginhoux F, Ng LG. Combinatorial single-cell analyses of granulocyte-monocyte progenitor heterogeneity reveals an early uni-potent neutrophil progenitor. Immunity 2020;53:303–318.e305. [DOI] [PubMed] [Google Scholar]
- 14. Dinh HQ, Eggert T, Meyer MA, Zhu YP, Olingy CE, Llewellyn R, Wu R, Hedrick CC. Coexpression of CD71 and CD117 identifies an early unipotent neutrophil progenitor population in human bone marrow. Immunity 2020;53:319–334.e316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grassi L, Pourfarzad F, Ullrich S, Merkel A, Were F, Carrillo-de-Santa-Pau E, Yi G, Hiemstra IH, Tool ATJ, Mul E, Perner J, Janssen-Megens E, Berentsen K, Kerstens H, Habibi E, Gut M, Yaspo ML, Linser M, Lowy E, Datta A, Clarke L, Flicek P, Vingron M, Roos D, van den Berg TK, Heath S, Rico D, Frontini M, Kostadima M, Gut I, Valencia A, Ouwehand WH, Stunnenberg HG, Martens JHA, Kuijpers TW. Dynamics of transcription regulation in human bone marrow myeloid differentiation to mature blood neutrophils. Cell Rep 2018;24:2784–2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Katakura F, Nishiya K, Wentzel AS, Hino E, Miyamae J, Okano M, Wiegertjes GF, Moritomo T. Paralogs of common carp granulocyte colony-stimulating factor (G-CSF) have different functions regarding development, trafficking and activation of neutrophils. Front Immunol 2019;10:255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Soehnlein O, Steffens S, Hidalgo A, Weber C. Neutrophils as protagonists and targets in chronic inflammation. Nat Rev Immunol 2017;17:248–261. [DOI] [PubMed] [Google Scholar]
- 18. Bajrami B, Zhu H, Kwak HJ, Mondal S, Hou Q, Geng G, Karatepe K, Zhang YC, Nombela-Arrieta C, Park SY, Loison F, Sakai J, Xu Y, Silberstein LE, Luo HR. G-CSF maintains controlled neutrophil mobilization during acute inflammation by negatively regulating CXCR2 signaling. J Exp Med 2016;213:1999–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Basu S, Hodgson G, Zhang HH, Katz M, Quilici C, Dunn AR. "Emergency" granulopoiesis in G-CSF-deficient mice in response to Candida albicans infection. Blood 2000;95:3725–3733. [PubMed] [Google Scholar]
- 20. Gasparetto C, Laver J, Abboud M, Gillio A, Smith C, O'Reilly RJ, Moore MA. Effects of interleukin-1 on hematopoietic progenitors: evidence of stimulatory and inhibitory activities in a primate model. Blood 1989;74:547–550. [PubMed] [Google Scholar]
- 21. Manz MG, Boettcher S. Emergency granulopoiesis. Nat Rev Immunol 2014;14:302–314. [DOI] [PubMed] [Google Scholar]
- 22. Hibbs ML, Quilici C, Kountouri N, Seymour JF, Armes JE, Burgess AW, Dunn AR. Mice lacking three myeloid colony-stimulating factors (G-CSF, GM-CSF, and M-CSF) still produce macrophages and granulocytes and mount an inflammatory response in a sterile model of peritonitis. J Immunol 2007;178:6435–6443. [DOI] [PubMed] [Google Scholar]
- 23. Athens JW, Raab SO, Haab OP, Mauer AM, Ashenbrucker H, Cartwright GE, Wintrobe MM. Leukokinetic studies. III. The distribution of granulocytes in the blood of normal subjects. J Clin Invest 1961;40:159–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Weber PS, Toelboell T, Chang LC, Tirrell JD, Saama PM, Smith GW, Burton JL. Mechanisms of glucocorticoid-induced down-regulation of neutrophil L-selectin in cattle: evidence for effects at the gene-expression level and primarily on blood neutrophils. J Leukoc Biol 2004;75:815–827. [DOI] [PubMed] [Google Scholar]
- 25. Peschon JJ, Slack JL, Reddy P, Stocking KL, Sunnarborg SW, Lee DC, Russell WE, Castner BJ, Johnson RS, Fitzner JN, Boyce RW, Nelson N, Kozlosky CJ, Wolfson MF, Rauch CT, Cerretti DP, Paxton RJ, March CJ, Black RA. An essential role for ectodomain shedding in mammalian development. Science 1998;282:1281–1284. [DOI] [PubMed] [Google Scholar]
- 26. Preece G, Murphy G, Ager A. Metalloproteinase-mediated regulation of L-selectin levels on leucocytes. J Biol Chem 1996;271:11634–11640. [DOI] [PubMed] [Google Scholar]
- 27. Fay ME, Myers DR, Kumar A, Turbyfield CT, Byler R, Crawford K, Mannino RG, Laohapant A, Tyburski EA, Sakurai Y, Rosenbluth MJ, Switz NA, Sulchek TA, Graham MD, Lam WA. Cellular softening mediates leukocyte demargination and trafficking, thereby increasing clinical blood counts. Proc Natl Acad Sci USA 2016;113:1987–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lavkan AH, Astiz ME, Rackow EC. Effects of proinflammatory cytokines and bacterial toxins on neutrophil rheologic properties. Crit Care Med 1998;26:1677–1682. [DOI] [PubMed] [Google Scholar]
- 29. Suratt BT, Young SK, Lieber J, Nick JA, Henson PM, Worthen GS. Neutrophil maturation and activation determine anatomic site of clearance from circulation. Am J Physiol Lung Cell Mol Physiol 2001;281:L913–L921. [DOI] [PubMed] [Google Scholar]
- 30. Rotzius P, Thams S, Soehnlein O, Kenne E, Tseng CN, Bjorkstrom NK, Malmberg KJ, Lindbom L, Eriksson EE. Distinct infiltration of neutrophils in lesion shoulders in ApoE-/- mice. Am J Pathol 2010;177:493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yvan-Charvet L, Ranalletta M, Wang N, Han S, Terasaka N, Li R, Welch C, Tall AR. Combined deficiency of ABCA1 and ABCG1 promotes foam cell accumulation and accelerates atherosclerosis in mice. J Clin Invest 2007;117:3900–3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Murphy AJ, Akhtari M, Tolani S, Pagler T, Bijl N, Kuo CL, Wang M, Sanson M, Abramowicz S, Welch C, Bochem AE, Kuivenhoven JA, Yvan-Charvet L, Tall AR. ApoE regulates hematopoietic stem cell proliferation, monocytosis, and monocyte accumulation in atherosclerotic lesions in mice. J Clin Invest 2011;121:4138–4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Westerterp M, Gourion-Arsiquaud S, Murphy AJ, Shih A, Cremers S, Levine RL, Tall AR, Yvan-Charvet L. Regulation of hematopoietic stem and progenitor cell mobilization by cholesterol efflux pathways. Cell Stem Cell 2012;11:195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pelton K, Krieder J, Joiner D, Freeman MR, Goldstein SA, Solomon KR. Hypercholesterolemia promotes an osteoporotic phenotype. Am J Pathol 2012;181:928–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Klimek E, Mikołajczyk T, Sulicka J, Kwaśny-Krochin B, Korkosz M, Osmenda G, Wizner B, Surdacki A, Guzik T, Grodzicki TK, Skalska A. Blood monocyte subsets and selected cardiovascular risk markers in rheumatoid arthritis of short duration in relation to disease activity. Biomed Res Int 2014;2014:736853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sargin G, Senturk T, Yavasoglu I, Kose R. Relationship between neutrophil-lymphocyte, platelet-lymphocyte ratio and disease activity in rheumatoid arthritis treated with rituximab. Int J Rheum Dis 2018;21:2122–2127. [DOI] [PubMed] [Google Scholar]
- 37. Dragoljevic D, Kraakman MJ, Nagareddy PR, Ngo D, Shihata W, Kammoun HL, Whillas A, Lee MKS, Al-Sharea A, Pernes G, Flynn MC, Lancaster GI, Febbraio MA, Chin-Dusting J, Hanaoka BY, Wicks IP, Murphy AJ. Defective cholesterol metabolism in haematopoietic stem cells promotes monocyte-driven atherosclerosis in rheumatoid arthritis. Eur Heart J 2018;39:2158–2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nagareddy PR, Murphy AJ, Stirzaker RA, Hu Y, Yu S, Miller RG, Ramkhelawon B, Distel E, Westerterp M, Huang LS, Schmidt AM, Orchard TJ, Fisher EA, Tall AR, Goldberg IJ. Hyperglycemia promotes myelopoiesis and impairs the resolution of atherosclerosis. Cell Metab 2013;17:695–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Flynn MC, Kraakman MJ, Tikellis C, Lee MKS, Hanssen NMJ, Kammoun HL, Pickering RJ, Dragoljevic D, Al-Sharea A, Barrett TJ, Hortle F, Byrne FL, Olzomer E, McCarthy DA, Schalkwijk CG, Forbes JM, Hoehn K, Makowski L, Lancaster GI, El-Osta A, Fisher EA, Goldberg IJ, Cooper ME, Nagareddy PR, Thomas MC, Murphy AJ. Transient intermittent hyperglycemia accelerates atherosclerosis by promoting myelopoiesis. Circ Res 2020;127:877–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schmidt MI, Duncan BB, Sharrett AR, Lindberg G, Savage PJ, Offenbacher S, Azambuja MI, Tracy RP, Heiss G. Markers of inflammation and prediction of diabetes mellitus in adults (Atherosclerosis Risk in Communities study): a cohort study. Lancet 1999;353:1649–1652. [DOI] [PubMed] [Google Scholar]
- 41. Pietras EM, Mirantes-Barbeito C, Fong S, Loeffler D, Kovtonyuk LV, Zhang SYi, Lakshminarasimhan R, Chin CP, Techner J-M, Will B, Nerlov C, Steidl U, Manz MG, Schroeder T, Passegué E. Chronic interleukin-1 exposure drives haematopoietic stem cells towards precocious myeloid differentiation at the expense of self-renewal. Nat Cell Biol 2016;18:607–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tyrkalska SD, Pérez-Oliva AB, Rodríguez-Ruiz L, Martínez-Morcillo FJ, Alcaraz-Pérez F, Martínez-Navarro FJ, Lachaud C, Ahmed N, Schroeder T, Pardo-Sánchez I, Candel S, López-Muñoz A, Choudhuri A, Rossmann MP, Zon LI, Cayuela ML, García-Moreno D, Mulero V. Inflammasome regulates hematopoiesis through cleavage of the master erythroid transcription factor GATA1. Immunity 2019;51:50–63.e55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Christ A, Günther P, Lauterbach MAR, Duewell P, Biswas D, Pelka K, Scholz CJ, Oosting M, Haendler K, Baßler K, Klee K, Schulte-Schrepping J, Ulas T, Moorlag SJCFM, Kumar V, Park MH, Joosten LAB, Groh LA, Riksen NP, Espevik T, Schlitzer A, Li Y, Fitzgerald ML, Netea MG, Schultze JL, Latz E. Western diet triggers NLRP3-dependent innate immune reprogramming. Cell 2018;172:162–175.e114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nagareddy PR, Asfour A, Klyachkin YM, Abdel-Latif A. A novel role for bioactive lipids in stem cell mobilization during cardiac ischemia: new paradigms in thrombosis: novel mediators and biomarkers. J Thromb Thrombolysis 2014;37:24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Talukdar S, Oh DY, Bandyopadhyay G, Li D, Xu J, McNelis J, Lu M, Li P, Yan Q, Zhu Y, Ofrecio J, Lin M, Brenner MB, Olefsky JM. Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nat Med 2012;18:1407–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hanssen NMJ, Spaetgens B, Nagareddy PR, Murphy AJ. DAMPening mortality in COVID-19: therapeutic insights from basic cardiometabolic studies on S100A8/A9. Circulation 2021;143:971–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cappuccio FP, Cooper D, D'Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J 2011;32:1484–1492. [DOI] [PubMed] [Google Scholar]
- 48. McAlpine CS, Kiss MG, Rattik S, He S, Vassalli A, Valet C, Anzai A, Chan CT, Mindur JE, Kahles F, Poller WC, Frodermann V, Fenn AM, Gregory AF, Halle L, Iwamoto Y, Hoyer FF, Binder CJ, Libby P, Tafti M, Scammell TE, Nahrendorf M, Swirski FK. Sleep modulates haematopoiesis and protects against atherosclerosis. Nature 2019;566:383–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Geovanini GR, Wang R, Weng J, Tracy R, Jenny NS, Goldberger AL, Costa MD, Liu Y, Libby P, Redline S. Elevations in neutrophils with obstructive sleep apnea: the Multi-Ethnic Study of Atherosclerosis (MESA). Int J Cardiol 2018;257:318–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Poller WC, Nahrendorf M, Swirski FK. Hematopoiesis and cardiovascular disease. Circ Res 2020;126:1061–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dutta P, Courties G, Wei Y, Leuschner F, Gorbatov R, Robbins CS, Iwamoto Y, Thompson B, Carlson AL, Heidt T, Majmudar MD, Lasitschka F, Etzrodt M, Waterman P, Waring MT, Chicoine AT, van der Laan AM, Niessen HW, Piek JJ, Rubin BB, Butany J, Stone JR, Katus HA, Murphy SA, Morrow DA, Sabatine MS, Vinegoni C, Moskowitz MA, Pittet MJ, Libby P, Lin CP, Swirski FK, Weissleder R, Nahrendorf M. Myocardial infarction accelerates atherosclerosis. Nature 2012;487:325–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Courties G, Herisson F, Sager HB, Heidt T, Ye Y, Wei Y, Sun Y, Severe N, Dutta P, Scharff J, Scadden DT, Weissleder R, Swirski FK, Moskowitz MA, Nahrendorf M. Ischemic stroke activates hematopoietic bone marrow stem cells. Circ Res 2015;116:407–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Peshkova IO, Aghayev T, Fatkhullina AR, Makhov P, Titerina EK, Eguchi S, Tan YF, Kossenkov AV, Khoreva MV, Gankovskaya LV, Sykes SM, Koltsova EK. IL-27 receptor-regulated stress myelopoiesis drives abdominal aortic aneurysm development. Nat Commun 2019;10:5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Santisteban MM, Kim S, Pepine CJ, Raizada MK. Brain-gut-bone marrow axis: implications for hypertension and related therapeutics. Circ Res 2016;118:1327–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Al-Sharea A, Lee MKS, Purton LE, Hawkins ED, Murphy AJ. The haematopoietic stem cell niche: a new player in cardiovascular disease? Cardiovasc Res 2019;115:277–291. [DOI] [PubMed] [Google Scholar]
- 56. Kim S, Zingler M, Harrison JK, Scott EW, Cogle CR, Luo D, Raizada MK. Angiotensin II regulation of proliferation, differentiation, and engraftment of hematopoietic stem cells. Hypertension 2016;67:574–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Johns JL, Christopher MM. Extramedullary hematopoiesis: a new look at the underlying stem cell niche, theories of development, and occurrence in animals. Vet Pathol 2012;49:508–523. [DOI] [PubMed] [Google Scholar]
- 58. Pinho S, Frenette PS. Haematopoietic stem cell activity and interactions with the niche. Nat Rev Mol Cell Biol 2019;20:303–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Steensma DP, Bejar R, Jaiswal S, Lindsley RC, Sekeres MA, Hasserjian RP, Ebert BL. Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood 2015;126:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Fuster JJ, MacLauchlan S, Zuriaga MA, Polackal MN, Ostriker AC, Chakraborty R, Wu CL, Sano S, Muralidharan S, Rius C, Vuong J, Jacob S, Muralidhar V, Robertson AA, Cooper MA, Andres V, Hirschi KK, Martin KA, Walsh K. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science 2017;355:842–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sano S, Oshima K, Wang Y, MacLauchlan S, Katanasaka Y, Sano M, Zuriaga MA, Yoshiyama M, Goukassian D, Cooper MA, Fuster JJ, Walsh K. Tet2-mediated clonal hematopoiesis accelerates heart failure through a mechanism involving the IL-1beta/NLRP3 inflammasome. J Am Coll Cardiol 2018;71:875–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wang W, Liu W, Fidler T, Wang Y, Tang Y, Woods B, Welch C, Cai B, Silvestre-Roig C, Ai D, Yang YG, Hidalgo A, Soehnlein O, Tabas I, Levine RL, Tall AR, Wang N. Macrophage inflammation, erythrophagocytosis, and accelerated atherosclerosis in Jak2 (V617F) mice. Circ Res 2018;123:e35–e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wolach O, Sellar RS, Martinod K, Cherpokova D, McConkey M, Chappell RJ, Silver AJ, Adams D, Castellano CA, Schneider RK, Padera RF, DeAngelo DJ, Wadleigh M, Steensma DP, Galinsky I, Stone RM, Genovese G, McCarroll SA, Iliadou B, Hultman C, Neuberg D, Mullally A, Wagner DD, Ebert BL. Increased neutrophil extracellular trap formation promotes thrombosis in myeloproliferative neoplasms. Sci Transl Med 2018;10:eaan8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Stark MA, Huo Y, Burcin TL, Morris MA, Olson TS, Ley K. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity 2005;22:285–294. [DOI] [PubMed] [Google Scholar]
- 65. Smith E, Zarbock A, Stark MA, Burcin TL, Bruce AC, Foley P, Ley K. IL-23 is required for neutrophil homeostasis in normal and neutrophilic mice. J Immunol 2007;179:8274–8279. [DOI] [PubMed] [Google Scholar]
- 66. Zhang D, Frenette PS. Cross talk between neutrophils and the microbiota. Blood 2019;133:2168–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Silvestre-Roig C, Braster Q, Ortega-Gomez A, Soehnlein O. Neutrophils as regulators of cardiovascular inflammation. Nat Rev Cardiol 2020;17:327–340. [DOI] [PubMed] [Google Scholar]
- 68. Luo Y, Chen GL, Hannemann N, Ipseiz N, Kronke G, Bauerle T, Munos L, Wirtz S, Schett G, Bozec A. Microbiota from obese mice regulate hematopoietic stem cell differentiation by altering the bone niche. Cell Metab 2015;22:886–894. [DOI] [PubMed] [Google Scholar]
- 69. Brandsma E, Kloosterhuis NJ, Koster M, Dekker DC, Gijbels MJJ, van der Velden S, Rios-Morales M, van Faassen MJR, Loreti MG, de Bruin A, Fu J, Kuipers F, Bakker BM, Westerterp M, de Winther MPJ, Hofker MH, van de Sluis B, Koonen DPY. A proinflammatory gut microbiota increases systemic inflammation and accelerates atherosclerosis. Circ Res 2019;124:94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kain V, Van Der Pol W, Mariappan N, Ahmad A, Eipers P, Gibson DL, Gladine C, Vigor C, Durand T, Morrow C, Halade GV. Obesogenic diet in aging mice disrupts gut microbe composition and alters neutrophil:lymphocyte ratio, leading to inflamed milieu in acute heart failure. Faseb J 2019;33:6456–6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hanssen NMJ, de Vos WM, Nieuwdorp M. Fecal microbiota transplantation in human metabolic diseases: from a murky past to a bright future? Cell Metab 2021;33:1098–1110. [DOI] [PubMed] [Google Scholar]
- 72. Benakis C, Brea D, Caballero S, Faraco G, Moore J, Murphy M, Sita G, Racchumi G, Ling L, Pamer EG, Iadecola C, Anrather J. Commensal microbiota affects ischemic stroke outcome by regulating intestinal gammadelta T cells. Nat Med 2016;22:516–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Adrover JM, Del Fresno C, Crainiciuc G, Cuartero MI, Casanova-Acebes M, Weiss LA, Huerga-Encabo H, Silvestre-Roig C, Rossaint J, Cossío I, Lechuga-Vieco AV, García-Prieto J, Gómez-Parrizas M, Quintana JA, Ballesteros I, Martin-Salamanca S, Aroca-Crevillen A, Chong SZ, Evrard M, Balabanian K, López J, Bidzhekov K, Bachelerie F, Abad-Santos F, Muñoz-Calleja C, Zarbock A, Soehnlein O, Weber C, Ng LG, Lopez-Rodriguez C, Sancho D, Moro MA, Ibáñez B, Hidalgo A. A neutrophil timer coordinates immune defense and vascular protection. Immunity 2019;50:390–402.e310. [DOI] [PubMed] [Google Scholar]
- 74. Zhang D, Chen G, Manwani D, Mortha A, Xu C, Faith JJ, Burk RD, Kunisaki Y, Jang JE, Scheiermann C, Merad M, Frenette PS. Neutrophil ageing is regulated by the microbiome. Nature 2015;525:528–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Netea MG, Joosten LA, Latz E, Mills KH, Natoli G, Stunnenberg HG, O'Neill LA, Xavier RJ. Trained immunity: a program of innate immune memory in health and disease. Science 2016;352:aaf1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Quintin J, Saeed S, Martens JHA, Giamarellos-Bourboulis EJ, Ifrim DC, Logie C, Jacobs L, Jansen T, Kullberg BJ, Wijmenga C, Joosten LAB, Xavier RJ, van der Meer JWM, Stunnenberg HG, Netea MG. Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell Host Microbe 2012;12:223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Essers MA, Offner S, Blanco-Bose WE, Waibler Z, Kalinke U, Duchosal MA, Trumpp A. IFNalpha activates dormant haematopoietic stem cells in vivo. Nature 2009;458:904–908. [DOI] [PubMed] [Google Scholar]
- 78. Baldridge MT, King KY, Boles NC, Weksberg DC, Goodell MA. Quiescent haematopoietic stem cells are activated by IFN-gamma in response to chronic infection. Nature 2010;465:793–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Cremer S, Schloss MJ, Vinegoni C, Foy BH, Zhang S, Rohde D, Hulsmans M, Fumene Feruglio P, Schmidt S, Wojtkiewicz G, Higgins JM, Weissleder R, Swirski FK, Nahrendorf M. Diminished reactive hematopoiesis and cardiac inflammation in a mouse model of recurrent myocardial infarction. J Am Coll Cardiol 2020;75:901–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Murray CJ, Lopez AD. Measuring the global burden of disease. N Engl J Med 2013;369:448–457. [DOI] [PubMed] [Google Scholar]
- 81. Drechsler M, Megens RT, van Zandvoort M, Weber C, Soehnlein O. Hyperlipidemia-triggered neutrophilia promotes early atherosclerosis. Circulation 2010;122:1837–1845. [DOI] [PubMed] [Google Scholar]
- 82. Chiu JJ, Chien S. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol Rev 2011;91:327–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Zarbock A, Ley K. Mechanisms and consequences of neutrophil interaction with the endothelium. Am J Pathol 2008;172:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Rasmuson J, Kenne E, Wahlgren M, Soehnlein O, Lindbom L. Heparinoid sevuparin inhibits Streptococcus-induced vascular leak through neutralizing neutrophil-derived proteins. Faseb J 2019;33:10443–10452. [DOI] [PubMed] [Google Scholar]
- 85. Ortega-Gomez A, Salvermoser M, Rossaint J, Pick R, Brauner J, Lemnitzer P, Tilgner J, de Jong RJ, Megens RT, Jamasbi J, Döring Y, Pham CT, Scheiermann C, Siess W, Drechsler M, Weber C, Grommes J, Zarbock A, Walzog B, Soehnlein O. Cathepsin G controls arterial but not venular myeloid cell recruitment. Circulation 2016;134:1176–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Chèvre R, González-Granado JM, Megens RTA, Sreeramkumar V, Silvestre-Roig C, Molina-Sánchez P, Weber C, Soehnlein O, Hidalgo A, Andrés V. High-resolution imaging of intravascular atherogenic inflammation in live mice. Circ Res 2014;114:770–779. [DOI] [PubMed] [Google Scholar]
- 87. Eriksson EE. Intravital microscopy on atherosclerosis in apolipoprotein e-deficient mice establishes microvessels as major entry pathways for leukocytes to advanced lesions. Circulation 2011;124:2129–2138. [DOI] [PubMed] [Google Scholar]
- 88. Moreno PR, Purushothaman KR, Sirol M, Levy AP, Fuster V. Neovascularization in human atherosclerosis. Circulation 2006;113:2245–2252. [DOI] [PubMed] [Google Scholar]
- 89. Massena S, Christoffersson G, Vagesjo E, Seignez C, Gustafsson K, Binet F, Herrera Hidalgo C, Giraud A, Lomei J, Westrom S, Shibuya M, Claesson-Welsh L, Gerwins P, Welsh M, Kreuger J, Phillipson M. Identification and characterization of VEGF-A-responsive neutrophils expressing CD49d, VEGFR1, and CXCR4 in mice and humans. Blood 2015;126:2016–2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Hristov M, Zernecke A, Bidzhekov K, Liehn EA, Shagdarsuren E, Ludwig A, Weber C. Importance of CXC chemokine receptor 2 in the homing of human peripheral blood endothelial progenitor cells to sites of arterial injury. Circ Res 2007;100:590–597. [DOI] [PubMed] [Google Scholar]
- 91. Woodfin A, Voisin MB, Beyrau M, Colom B, Caille D, Diapouli FM, Nash GB, Chavakis T, Albelda SM, Rainger GE, Meda P, Imhof BA, Nourshargh S. The junctional adhesion molecule JAM-C regulates polarized transendothelial migration of neutrophils in vivo. Nat Immunol 2011;12:761–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Wang J, Hossain M, Thanabalasuriar A, Gunzer M, Meininger C, Kubes P. Visualizing the function and fate of neutrophils in sterile injury and repair. Science 2017;358:111–116. [DOI] [PubMed] [Google Scholar]
- 93. Zhang R, Lahens NF, Ballance HI, Hughes ME, Hogenesch JB. A circadian gene expression atlas in mammals: implications for biology and medicine. Proc Natl Acad Sci USA 2014;111:16219–16224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. de Juan A, Ince LM, Pick R, Chen C-S, Molica F, Zuchtriegel G, Wang C, Zhang D, Druzd D, Hessenauer MET, Pelli G, Kolbe I, Oster H, Prophete C, Hergenhan SM, Albrecht U, Ripperger J, Montanez E, Reichel CA, Soehnlein O, Kwak BR, Frenette PS, Scheiermann C. Associated sympathetic innervation drives rhythmic vascular inflammation of arteries and veins. Circulation 2019;140:1100–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Winter C, Silvestre-Roig C, Ortega-Gomez A, Lemnitzer P, Poelman H, Schumski A, Winter J, Drechsler M, de Jong R, Immler R, Sperandio M, Hristov M, Zeller T, Nicolaes GAF, Weber C, Viola JR, Hidalgo A, Scheiermann C, Soehnlein O. Chrono-pharmacological targeting of the CCL2-CCR2 axis ameliorates atherosclerosis. Cell Metab 2018;28:175–182.e175. [DOI] [PubMed] [Google Scholar]
- 96. Delporte C, Boudjeltia KZ, Noyon C, Furtmuller PG, Nuyens V, Slomianny MC, Madhoun P, Desmet JM, Raynal P, Dufour D, Koyani CN, Reye F, Rousseau A, Vanhaeverbeek M, Ducobu J, Michalski JC, Neve J, Vanhamme L, Obinger C, Malle E, Van Antwerpen P. Impact of myeloperoxidase-LDL interactions on enzyme activity and subsequent posttranslational oxidative modifications of apoB-100. J Lipid Res 2014;55:747–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Soehnlein O, Kai-Larsen Y, Frithiof R, Sorensen OE, Kenne E, Scharffetter-Kochanek K, Eriksson EE, Herwald H, Agerberth B, Lindbom L. Neutrophil primary granule proteins HBP and HNP1-3 boost bacterial phagocytosis by human and murine macrophages. J Clin Invest 2008;118:3491–3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Knight JS, Luo W, O'Dell AA, Yalavarthi S, Zhao W, Subramanian V, Guo C, Grenn RC, Thompson PR, Eitzman DT, Kaplan MJ. Peptidylarginine deiminase inhibition reduces vascular damage and modulates innate immune responses in murine models of atherosclerosis. Circ Res 2014;114:947–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Warnatsch A, Ioannou M, Wang Q, Papayannopoulos V. Inflammation. Neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science 2015;349:316–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Davies MJ. The pathophysiology of acute coronary syndromes. Heart 2000;83:361–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Mawhin MA, Tilly P, Zirka G, Charles AL, Slimani F, Vonesch JL, Michel JB, Back M, Norel X, Fabre JE. Neutrophils recruited by leukotriene B4 induce features of plaque destabilization during endotoxaemia. Cardiovasc Res 2018;114:1656–1666. [DOI] [PubMed] [Google Scholar]
- 102. Schumski A, Ortega-Gómez A, Wichapong K, Winter C, Lemnitzer P, Viola JR, Pinilla-Vera M, Folco E, Solis-Mezarino V, Völker-Albert M, Maas SL, Pan C, Perez Olivares L, Winter J, Hackeng T, Karlsson MCI, Zeller T, Imhof A, Baron RM, Nicolaes GAF, Libby P, Maegdefessel L, Kamp F, Benoit M, Döring Y, Soehnlein O. Endotoxinemia accelerates atherosclerosis through electrostatic charge-mediated monocyte adhesion. Circulation 2021;143:254–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Paulin N, Viola JR, Maas SL, de Jong R, Fernandes-Alnemri T, Weber C, Drechsler M, Döring Y, Soehnlein O. Double-strand DNA sensing Aim2 inflammasome regulates atherosclerotic plaque vulnerability. Circulation 2018;138:321–323. [DOI] [PubMed] [Google Scholar]
- 104. Jaiswal S, Natarajan P, Silver AJ, Gibson CJ, Bick AG, Shvartz E, McConkey M, Gupta N, Gabriel S, Ardissino D, Baber U, Mehran R, Fuster V, Danesh J, Frossard P, Saleheen D, Melander O, Sukhova GK, Neuberg D, Libby P, Kathiresan S, Ebert BL. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N Engl J Med 2017;377:111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Fidler TP, Xue C, Yalcinkaya M, Hardaway B, Abramowicz S, Xiao T, Liu W, Thomas DG, Hajebrahimi MA, Pircher J, Silvestre-Roig C, Kotini AG, Luchsinger LL, Wei Y, Westerterp M, Snoeck HW, Papapetrou EP, Schulz C, Massberg S, Soehnlein O, Ebert B, Levine RL, Reilly MP, Libby P, Wang N, Tall AR. The AIM2 inflammasome exacerbates atherosclerosis in clonal haematopoiesis. Nature 2021;592:296–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Arruda-Olson AM, Reeder GS, Bell MR, Weston SA, Roger VL. Neutrophilia predicts death and heart failure after myocardial infarction: a community-based study. Circ Cardiovasc Qual Outcomes 2009;2:656–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Nagareddy PR, Sreejit G, Abo-Aly M, Jaggers RM, Chelvarajan L, Johnson J, Pernes G, Athmanathan B, Abdel-Latif A, Murphy AJ. NETosis is required for S100A8/A9-induced granulopoiesis after myocardial infarction. Arterioscler Thromb Vasc Biol 2020;40:2805–2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Schloss MJ, Horckmans M, Nitz K, Duchene J, Drechsler M, Bidzhekov K, Scheiermann C, Weber C, Soehnlein O, Steffens S. The time-of-day of myocardial infarction onset affects healing through oscillations in cardiac neutrophil recruitment. EMBO Mol Med 2016;8:937–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Vasilyev N, Williams T, Brennan ML, Unzek S, Zhou X, Heinecke JW, Spitz DR, Topol EJ, Hazen SL, Penn MS. Myeloperoxidase-generated oxidants modulate left ventricular remodeling but not infarct size after myocardial infarction. Circulation 2005;112:2812–2820. [DOI] [PubMed] [Google Scholar]
- 110. Ali M, Pulli B, Courties G, Tricot B, Sebas M, Iwamoto Y, Hilgendorf I, Schob S, Dong A, Zheng W, Skoura A, Kalgukar A, Cortes C, Ruggeri R, Swirski FK, Nahrendorf M, Buckbinder L, Chen JW. Myeloperoxidase inhibition improves ventricular function and remodeling after experimental myocardial infarction. JACC Basic Transl Sci 2016;1:633–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Horckmans M, Ring L, Duchene J, Santovito D, Schloss MJ, Drechsler M, Weber C, Soehnlein O, Steffens S. Neutrophils orchestrate post-myocardial infarction healing by polarizing macrophages towards a reparative phenotype. Eur Heart J 2017;38:187–197. [DOI] [PubMed] [Google Scholar]
- 112. Fournier BM, Parkos CA. The role of neutrophils during intestinal inflammation. Mucosal Immunol 2012;5:354–366. [DOI] [PubMed] [Google Scholar]
- 113. Prabhu SD, Frangogiannis NG. The biological basis for cardiac repair after myocardial infarction: from inflammation to fibrosis. Circ Res 2016;119:91–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Marinkovic G, Koenis D, de Camp L, Jablonowski R, Graber N, de Waard V, de Vries CJ, Goncalves I, Nilsson J, Jovinge S, Schiopu A. S100A9 links inflammation and repair in myocardial infarction. Circ Res 2020;127:664–676. [DOI] [PubMed] [Google Scholar]
- 115. Hilgendorf I, Gerhardt LM, Tan TC, Winter C, Holderried TA, Chousterman BG, Iwamoto Y, Liao R, Zirlik A, Scherer-Crosbie M, Hedrick CC, Libby P, Nahrendorf M, Weissleder R, Swirski FK. Ly-6Chigh monocytes depend on Nr4a1 to balance both inflammatory and reparative phases in the infarcted myocardium. Circ Res 2014;114:1611–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Howangyin KY, Zlatanova I, Pinto C, Ngkelo A, Cochain C, Rouanet M, Vilar J, Lemitre M, Stockmann C, Fleischmann BK, Mallat Z, Silvestre JS. Myeloid-epithelial-reproductive receptor tyrosine kinase and milk fat globule epidermal growth factor 8 coordinately improve remodeling after myocardial infarction via local delivery of vascular endothelial growth factor. Circulation 2016;133:826–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Ma Y, Yabluchanskiy A, Iyer RP, Cannon PL, Flynn ER, Jung M, Henry J, Cates CA, Deleon-Pennell KY, Lindsey ML. Temporal neutrophil polarization following myocardial infarction. Cardiovasc Res 2016;110:51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Lörchner H, Pöling J, Gajawada P, Hou Y, Polyakova V, Kostin S, Adrian-Segarra JM, Boettger T, Wietelmann A, Warnecke H, Richter M, Kubin T, Braun T. Myocardial healing requires Reg3β-dependent accumulation of macrophages in the ischemic heart. Nat Med 2015;21:353–362. [DOI] [PubMed] [Google Scholar]
- 119. Ferraro B, Leoni G, Hinkel R, Ormanns S, Paulin N, Ortega-Gomez A, Viola JR, de Jong R, Bongiovanni D, Bozoglu T, Maas SL, D'Amico M, Kessler T, Zeller T, Hristov M, Reutelingsperger C, Sager HB, Doring Y, Nahrendorf M, Kupatt C, Soehnlein O. Pro-angiogenic macrophage phenotype to promote myocardial repair. J Am Coll Cardiol 2019;73:2990–3002. [DOI] [PubMed] [Google Scholar]
- 120. García-Prieto J, Villena-Gutiérrez R, Gómez M, Bernardo E, Pun-García A, García-Lunar I, Crainiciuc G, Fernández-Jiménez R, Sreeramkumar V, Bourio-Martínez R, García-Ruiz JM, Del Valle AS, Sanz-Rosa D, Pizarro G, Fernández-Ortiz A, Hidalgo A, Fuster V, Ibanez B. Neutrophil stunning by metoprolol reduces infarct size. Nat Commun 2017;8:14780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Ibanez B, Macaya C, Sánchez-Brunete V, Pizarro G, Fernández-Friera L, Mateos A, Fernández-Ortiz A, García-Ruiz JM, García-Álvarez A, Iñiguez A, Jiménez-Borreguero J, López-Romero P, Fernández-Jiménez R, Goicolea J, Ruiz-Mateos B, Bastante T, Arias M, Iglesias-Vázquez JA, Rodriguez MD, Escalera N, Acebal C, Cabrera JA, Valenciano J, Pérez de Prado A, Fernández-Campos MJ, Casado I, García-Rubira JC, García-Prieto J, Sanz-Rosa D, Cuellas C, Hernández-Antolín R, Albarrán A, Fernández-Vázquez F, de la Torre-Hernández JM, Pocock S, Sanz G, Fuster V. Effect of early metoprolol on infarct size in ST-segment-elevation myocardial infarction patients undergoing primary percutaneous coronary intervention: the Effect of Metoprolol in Cardioprotection During an Acute Myocardial Infarction (METOCARD-CNIC) Trial. Circulation 2013;128:1495–1503. [DOI] [PubMed] [Google Scholar]
- 122. Marinkovic G, Grauen Larsen H, Yndigegn T, Szabo IA, Mares RG, de Camp L, Weiland M, Tomas L, Goncalves I, Nilsson J, Jovinge S, Schiopu A. Inhibition of pro-inflammatory myeloid cell responses by short-term S100A9 blockade improves cardiac function after myocardial infarction. Eur Heart J 2019;40:2713–2723. [DOI] [PubMed] [Google Scholar]
- 123. Liberale L, Montecucco F, Schwarz L, Luscher TF, Camici GG. Inflammation and cardiovascular diseases: lessons from seminal clinical trials. Cardiovasc Res 2021;117:411–422. [DOI] [PubMed] [Google Scholar]
- 124. Braunersreuther V, Pellieux C, Pelli G, Burger F, Steffens S, Montessuit C, Weber C, Proudfoot A, Mach F, Arnaud C. Chemokine CCL5/RANTES inhibition reduces myocardial reperfusion injury in atherosclerotic mice. J Mol Cell Cardiol 2010;48:789–798. [DOI] [PubMed] [Google Scholar]
- 125. EU Clinical Trials Register . Clinical Trials Register. 2016. (https://www.clinicaltrialsregister.eu/ctr-search/trial/2016-000775-24/GB).
- 126. Sreejit G, Flynn MC, Patil M, Krishnamurthy P, Murphy AJ, Nagareddy PR. S100 family proteins in inflammation and beyond. Adv Clin Chem 2020;98:173–231. [DOI] [PubMed] [Google Scholar]
- 127. Abderrazak A, Couchie D, Mahmood DF, Elhage R, Vindis C, Laffargue M, Mateo V, Buchele B, Ayala MR, El Gaafary M, Syrovets T, Slimane MN, Friguet B, Fulop T, Simmet T, El Hadri K, Rouis M. Anti-inflammatory and antiatherogenic effects of the NLRP3 inflammasome inhibitor arglabin in ApoE2.Ki mice fed a high-fat diet. Circulation 2015;131:1061–1070. [DOI] [PubMed] [Google Scholar]
- 128. van Hout GP, Bosch L, Ellenbroek GH, de Haan JJ, van Solinge WW, Cooper MA, Arslan F, de Jager SC, Robertson AA, Pasterkamp G, Hoefer IE. The selective NLRP3-inflammasome inhibitor MCC950 reduces infarct size and preserves cardiac function in a pig model of myocardial infarction. Eur Heart J 2017;38:828–836. [DOI] [PubMed] [Google Scholar]
- 129. Franck G, Mawson TL, Folco EJ, Molinaro R, Ruvkun V, Engelbertsen D, Liu X, Tesmenitsky Y, Shvartz E, Sukhova GK, Michel JB, Nicoletti A, Lichtman A, Wagner D, Croce KJ, Libby P. Roles of PAD4 and NETosis in experimental atherosclerosis and arterial injury: implications for superficial erosion. Circ Res 2018;123:33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Rossaint J, Herter JM, Van Aken H, Napirei M, Doring Y, Weber C, Soehnlein O, Zarbock A. Synchronized integrin engagement and chemokine activation is crucial in neutrophil extracellular trap-mediated sterile inflammation. Blood 2014;123:2573–2584. [DOI] [PubMed] [Google Scholar]
- 131. Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol 2018;18:134–147. [DOI] [PubMed] [Google Scholar]