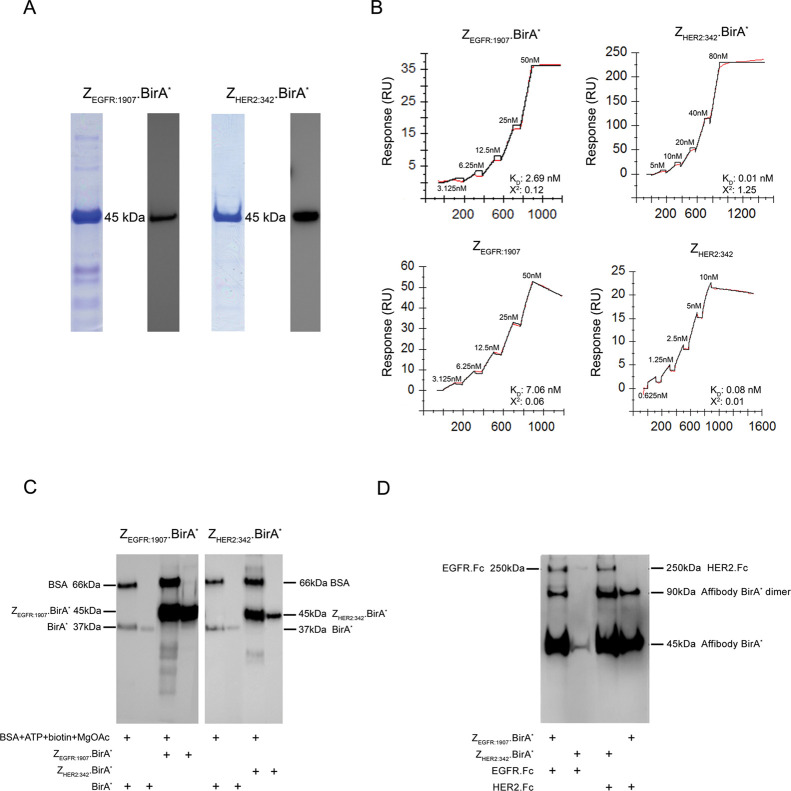
Figure 3.
ZEGFR:1907.BirA* and ZHER2:34.BirA* are able to retain their binding and biotinylation activities. (A) ZEGFR:1907.BirA* and ZHER2:342.BirA* were expressed as described in the Methods section, and their purity and size confirmed by SDS-PAGE and by Western blot using an anti-Histidine tag antibody. Both ZEGFR:1907.BirA* and ZHER2:342.BirA* migrate as a single 45 kDa band in the presence of 50 mM DTT (reducing conditions). (B) Single-cycle kinetics sensorgrams derived from increasing concentrations of ZEGFR:1907.BirA* and ZHER2:342.BirA* interacting with their respective immobilized targets [EGFR and HER2], as monitored using SPR. The binding affinity of each Affibody.BirA* construct was compared to that of its corresponding affibody alone. The binding of ZEGFR:1907.BirA* and ZEGFR:1907 to HER2 and ZHER2:342.BirA* and ZHER2:342 to EGFR served as negative controls to confirm the specificity of each Affibody.BirA* construct for their target (Supplementary Figure 1). (C) Monitoring the biotinylation activity of ZEGFR:1907.BirA*, ZHER2:342.BirA*, and BirA* alone toward BSA by Western blot analysis in the presence of ATP, biotin, and MgOAc.BirA*, ZEGFR:1907.BirA*, and ZHER2:342.BirA* are also biotinylated during the reaction. The Western blot samples were prepared in 50 mM DTT (reducing conditions). (D) Monitoring the biotinylation activity of ZEGFR:1907.BirA* and ZHER2:342.BirA* toward their recombinant target human EGFR.Fc and HER2.Fc, respectively, by Western blot analysis. ZEGFR:1907.BirA* and ZEGFR:1907 were allowed to bind and biotinylate each immobilized target, serving as both negative and positive controls to confirm the specificity of each Affibody.BirA* construct for their respective target. ZEGFR:1907.BirA* and ZHER2:342.BirA* were also biotinylated during this reaction. No reducing agent was added to the samples in panel D to maintain EGFR.Fc and HER2.Fc as dimers. As such, Affibody.BirA* constructs can exist as monomers and as disulfide bridge-containing dimers.