Fig. 6. β-Arrestin is not necessary for CXCR3-dependent ERK activation, and no Gαi:β-arrestin:ERK complex is observed.
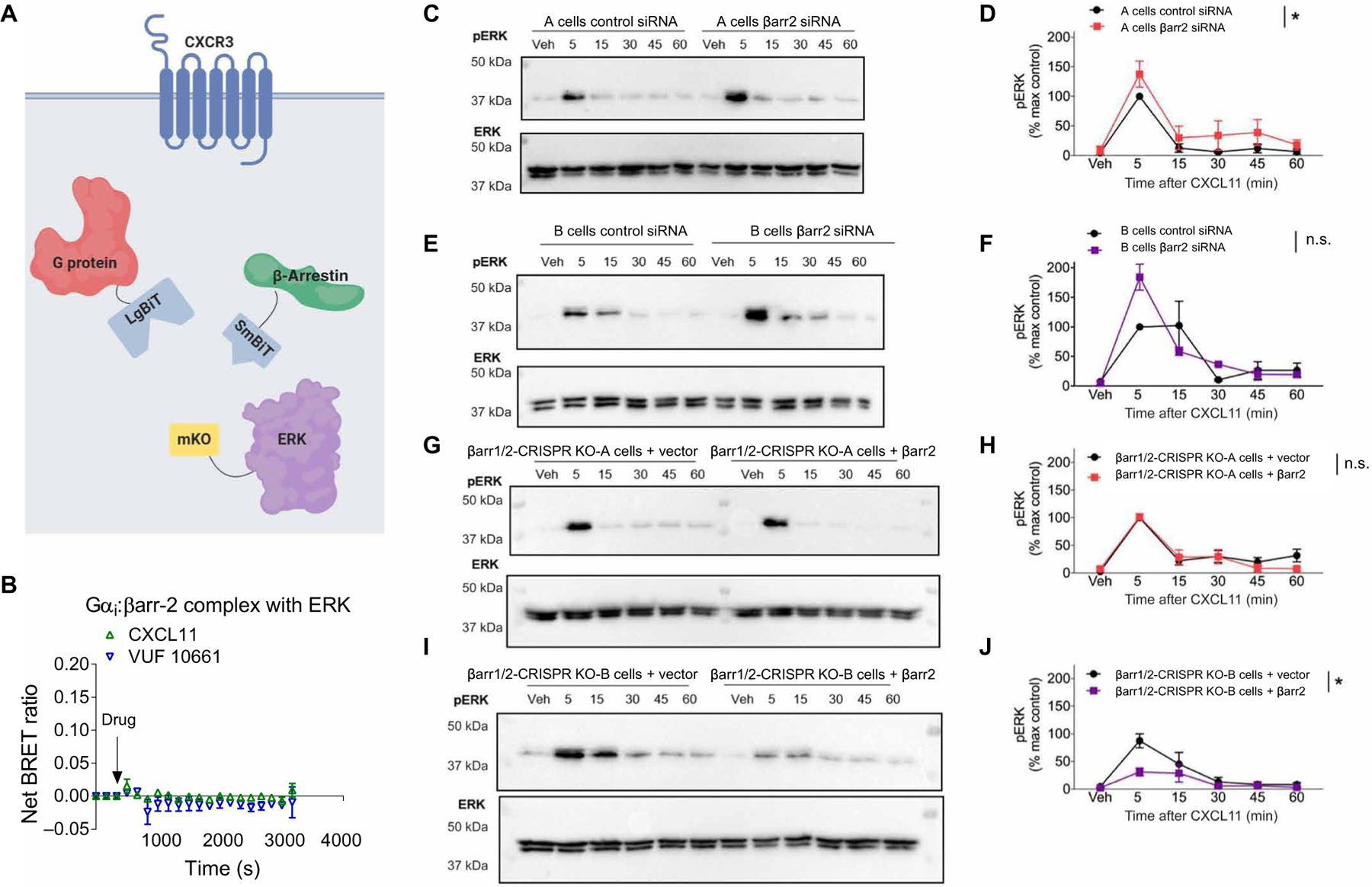
(A) Arrangement of the luciferase fragments and mKO acceptor fluorophore for complex BRET on Gαi (LgBiT), β-arrestin2 (SmBiT), and ERK2 (mKO). (B) Complex BRET ratio for Gαi:β-arrestin2:ERK after treatment with 100 nM CXCL11, 1 μM VUF10661, or vehicle. Data were normalized to both vehicle treatment and cytosolic mKO. (C to G) Western blotting analysis of the time course of ERK phosphorylation in A and B parental and β-arrestin1/2 CRISPR KO HEK293 cells stimulated with 100 nM CXCL11. (C) Western blotting analysis of phospho-ERK (pERK) and (D) its quantification in A parental cells transfected with control siRNA or β-arrestin2–specific siRNA. (E) Western blotting analysis of phospho-ERK and (F) its quantification in B parental cells transfected with control siRNA or β-arrestin2–specific siRNA. (G) Western blotting analysis of phospho-ERK and (H) its quantification in A β-arrestin1/2 CRISPR KO cells transfected with the control or β-arrestin2 rescue plasmid. (I) Western blotting analysis of phospho-ERK and (J) its quantification in B β-arrestin1/2 CRISPR KO cells transfected with the control or β-arrestin2 rescue plasmid. *P < 0.05 by two-way ANOVA to determine the main effect of either the siRNA or rescue. Data are from three experiments per condition. n.s., not significant.
