Stroke and peripheral thromboembolism related to atrial fibrillation (AF) represent a major medical and socio-economic problem worldwide. The left atrial appendage (LAA) is considered the main reservoir for thrombus formation in patients with AF and therefore eliminating it has been attempted for more than 50 years1. The first-in-human percutaneous occlusion of the LAA with a dedicated device was performed in August 2001 by Horst Sievert in Frankfurt, Germany, using the (now discontinued) PLAATO device2,3. The most widely used devices since then have been the WATCHMAN™️ device (Boston Scientific, Marlborough, MA, USA), the AMPLATZER™️ Cardiac Plug (ACP) and its successor, the AMPLATZER Amulet™️ device (both by Abbott Vascular, Santa Clara, CA, USA), and more recently the LAmbre™️ device (Lifetech Scientific Co, Ltd, Shenzhen, China)4. In this issue of AsiaIntervention, Cheung et al present a series of Chinese patients who were treated (in Hong Kong) with the WATCHMAN, ACP, Amulet or LAmbre device5.
This is an observational non-randomised study so direct device comparisons cannot be made. However, it is a valuable report of real-world LAAO therapy with four different devices that includes the learning curve effects and technical and procedural evolutionary steps in the field. Moreover, it is one of the few studies performed in a Chinese population, which makes it extremely valuable for the development of LAAO therapy in Asia.
The original quote of Hippocrates regarding the role of a physician «ἀσκεῖν περὶ τὰ νοσήματα δύο, ὠφελεῖν ἢ μὴ βλάπτειν» or “Primum non nocere” in Latin or “First do no harm” in English (看邱꼇狼隣냥傷벧 in Chinese) applies perfectly to AF-related stroke prevention therapies. Finding the fine balance between medication/intervention efficacy and safety, i.e., stroke prevention versus bleeding for oral anticoagulation (OAC) therapy, and stroke prevention versus procedure-related complications for LAAO, is surely the path to success6. The current gold standard OAC therapy is limited by a number of factors such as bleeding, poor patient compliance, cost, etc. The driving force for the development of percutaneous LAAO is the patient’s need for a minimally invasive, safe, “local” therapy for reducing their risk of AF-related thromboembolism, without suffering from major or minor bleeding problems. Therefore, there is a need for LAAO; the question is how to respond to it and how to become skilled in doing so.
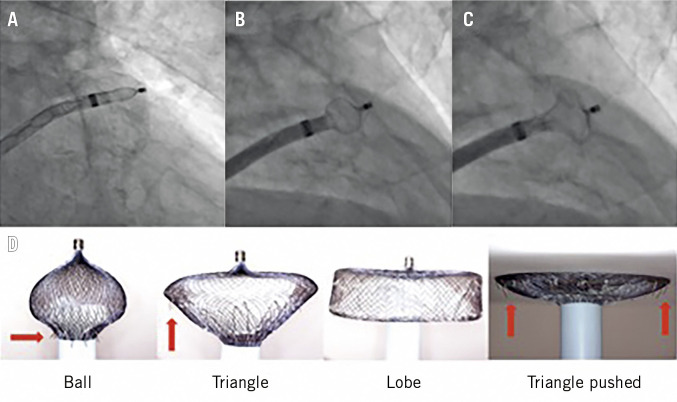
The biggest technical challenges of percutaneous LAAO therapy are the highly variable LAA anatomy, the fact that the LAA tissue is very fragile, and (for less experienced operators) the need for transseptal access. Preprocedural imaging with good quality transoesophageal echocardiography and/or cardiac computed tomography (CT) (or 3D printing) has been recognised as an important factor for procedural success, in an analogy of transcatheter aortic and mitral valve procedures. Advances in and technical improvement of the LAAO technique per se are also highly important. For example, the use of a pigtail catheter to access the LAA has decreased the pericardial bleeding events with the WATCHMAN device, whereas the use of the “ball” and “triangle” shape techniques has led to optimised and safer LAA implants with the ACP or the Amulet device (Figure 1)7.
Figure 1. Evolutionary steps in safer deployment of the AMPLATZER Cardiac Plug and the AMPLATZER Amulet device.
“Unsheathing” in the left atrial appendage (A) to form a “ball” to enter (B) to create a “triangle” for harmless device pushing in shallow anatomies (C). Gradual and controlled pushing of the delivery wire forms the Amulet lobe in different ways and shapes (D). The “ball” shape is used for initial engagement and rotation/orientation of the sheath as the stabilising wires (red arrows) are close to the sheath and cannot damage the LAA wall, whereas the “triangle” shape is used to push against the LAA wall as the distal lobe pin is retracted inside the lobe, but without rotating the device as the stabilising wires are now exposed (red arrows).
Another important challenge of LAAO therapy that is often not adequately discussed is the patient’s actual needs and characteristics. For example, the most common indication for LAAO in the study of Cheung et al is poor compliance to OAC therapy, whereas in the vast majority of European or American studies the principal indication is previous major bleeding and high bleeding risk6. A patient with poor compliance may not have a high bleeding risk at all so procedural and post-procedure antithrombotic therapy may be very different compared to a patient who had a major bleeding and/or a stroke with documented LAA thrombus related to it. Knowing the patient is, therefore, crucial.
Before proceeding to decide which parts of LAAO therapies need further improvement, it is necessary to define all LAAO-related clinical and technical endpoints. The “Munich consensus document on definitions, endpoints, and data collection requirements for clinical studies” which was published in EuroIntervention in 2016 is a good tool for this purpose8. The relation between the presence and the degree of peri-device leaks and stroke is not yet fully understood. The rate of device thrombosis is fairly low and not clinically relevant in most studies; however, it is difficult to explain to a patient that a device that was implanted to prevent thromboembolism has developed a thrombus9. In any case, device design improvement, implant optimisation techniques, and structured operator teaching programmes are expected to tackle most of these issues in the near future.
In Asia, and particularly in China, LAAO therapy development needs to address a couple of different matters. As compared to Western countries, there is less compliance to OAC therapy due to lower patient education, increased medication cost, living in remote areas, etc.5,10. In addition, the reimbursement and cost of the LAAO procedure is different, with many patients having to pay out of their own pocket to get treated. In China, many of the LAAO procedures are combined with AF catheter ablation, in a “one stop shop” concept that may be clinically appealing but not yet fully investigated11. Finally, operator training and dissemination of technical expertise is another important challenge.
The number of centres engaging in LAAO therapy in Asia, and especially in China, is growing fast. Several very skilled operators perform a lot of procedures with optimal outcomes. Yet, the published data in the field are scarce. Hopefully, researchers will be encouraged by the efforts of Dr Cheung, the Prince of Wales physicians and their mentor and pioneer on LAAO, Yat Yin Lam, to publish more about LAAO in AsiaIntervention or another medical journal.
Acknowledgments
Conflict of interest statement
A. Tzikas is a clinical proctor for Abbott.
References
- Meier B, Stegink W, Tzikas A. History of Percutaneous Left Atrial Appendage Occlusion with AMPLATZER Devices. Interv Cardiol Clin. 2018;7:151–8. doi: 10.1016/j.iccl.2017.12.007. [DOI] [PubMed] [Google Scholar]
- Omran H, Tzikas A, Sievert H, Stock F. A History of Percutaneous Left Atrial Appendage Occlusion with the PLAATO Device. Interv Cardiol Clin. 2018;7:137–42. doi: 10.1016/j.iccl.2017.12.009. [DOI] [PubMed] [Google Scholar]
- Bayard YL, Omran H, Neuzil P, Thuesen L, Pichler M, Rowland E, Ramondo A, Ruzyllo W, Budts W, Montalescot G, Brugada P, Serruys PW, Vahanian A, Piéchaud JF, Bartorelli A, Marco J, Probst P, Kuck KH, Ostermayer SH, Büscheck F, Fischer E, Leetz M, Sievert H. PLAATO (Percutaneous Left Atrial Appendage Transcatheter Occlusion) for prevention of cardioembolic stroke in non-anticoagulation eligible atrial fibrillation patients: results from the European PLAATO study. EuroIntervention. 2010;6:220–6. [PubMed] [Google Scholar]
- Tzikas A. Evolving technologies for percutaneous left atrial appendage occlusion. Trends Cardiovasc Med. 2018 Sep 18; doi: 10.1016/j.tcm.2018.09.009. [DOI] [PubMed] [Google Scholar]
- Cheung GSH, So KCY, Chan CKY, Chan AKY, Lee APW, Lam YY, Yan BP. Comparison of three left atrial appendage occlusion devices for stroke prevention in patients with non-valvular atrial fibrillation: a single-centre seven-year experience with WATCHMAN, AMPLATZER Cardiac Plug/Amulet, LAmbre. AsiaIntervention. 2019;5:57–63. doi: 10.4244/AIJ-D-18-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzikas A, Bergmann MW. Left atrial appendage closure: patient, device and post-procedure drug selection. EuroIntervention. 2016;12 Suppl X:X48–54. doi: 10.4244/EIJV12SXA10. [DOI] [PubMed] [Google Scholar]
- Tzikas A, Gafoor S, Meerkin D, Freixa X, Cruz-Gonzalez I, Lewalter T, Saw J, Berti S, Nielsen-Kudsk JE, Ibrahim R, Lakkireddy D, Paul V, Arzamendi D, Nietlispach F, Worthley SG, Hildick-Smith D, Thambo JB, Tondo C, Aminian A, Kalarus Z, Schmidt B, Sondergaard L, Kefer J, Meier B, Park JW, Sievert H, Omran H. Left atrial appendage occlusion with the AMPLATZER Amulet device: an expert consensus step-by-step approach. EuroIntervention. 2016;11:1512–21. doi: 10.4244/EIJV11I13A292. [DOI] [PubMed] [Google Scholar]
- Lempereur M, Aminian A, Freixa X, Gafoor S, Kefer J, Tzikas A, Legrand V, Saw J. Device-associated thrombus formation after left atrial appendage occlusion: A systematic review of events reported with the Watchman, the Amplatzer Cardiac Plug and the Amulet. Catheter Cardiovasc Interv. 2017;90:E111–21. doi: 10.1002/ccd.26903. [DOI] [PubMed] [Google Scholar]
- Tzikas A, Holmes DR, Gafoor S, Ruiz CE, Blomström- Lundqvist, Diener HC, Cappato R, Kar S, Lee RJ, Byrne RA, Ibrahim R, Lakkireddy D, Soliman OI, Näbauer M, Schneider S, Brachmann J, Saver JL, Tiemann K, Sievert H, Camm AJ, Lewalter T. Percutaneous left atrial appendage occlusion: the Munich consensus document on definitions, endpoints, and data collection requirements for clinical studies. EuroIntervention. 2016;12:103–11. doi: 10.4244/EIJV12I1A18. [DOI] [PubMed] [Google Scholar]
- Wang ZZ, Du X, Wang W, Tang RB, Luo JG, Li C, Chang SS, Liu XH, Sang CH, Yu RH, Long DY, Wu JH, Bai R, Liu N, Ruan YF, Dong JZ, Ma CS. Long-Term Persistence of Newly Initiated Warfarin Therapy in Chinese Patients With Nonvalvular Atrial Fibrillation. Circ Cardiovasc Qual Outcomes. 2016;9:380–7. doi: 10.1161/CIRCOUTCOMES.115.002337. [DOI] [PubMed] [Google Scholar]
- Wintgens L, Romanov A, Phillips K, Ballesteros G, Swaans M, Folkeringa R, Garcia-Bolao I, Pokushalov E, Boersma L. Combined atrial fibrillation ablation and left atrial appendage closure: long-term follow-up from a large multicentre registry. Europace. 2018;20:1783–9. doi: 10.1093/europace/euy025. [DOI] [PubMed] [Google Scholar]