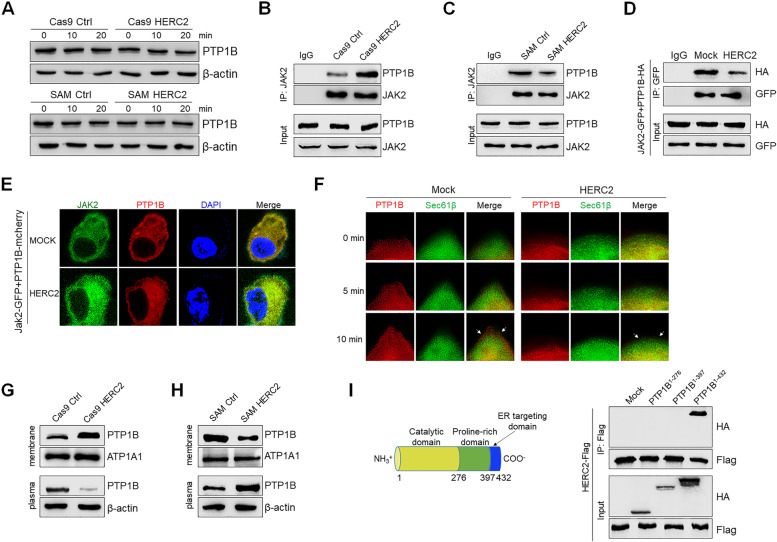
Fig. 7.
HERC2 inhibited PTP1B translocation in ER-PM junction. A HCC cells were treated with 50 ng/ml IL-6 for the indicated time points, and then the expression level of PTP1B was detected with western blot analysis. HERC2 knockout (B) and HERC2 overexpression (C) cells were treated with 50 ng/ml IL-6 for 20 min, and an immunoprecipitation assay was performed to analyze the interaction between JAK2 and PTP1B. D-E HEK293T cells were co-transfected with JAK2-GFP and PTP1B-HA (D) or PTP1B-mCherry (E) plasmids after being transfected with HERC2 or mock plasmids. The cells were then treated with 50 ng/ml IL-6 for 20 min. D An immunoprecipitation assay was performed to evaluate the interaction between JAK2 and PTP1B. E An immunofluorescence assay was performed to assess the colocalization of JAK2 with PTP1B. F HEK293T cells were co-transfected with PTP1B-mCherry and Sec61β-GFP plasmids. After transfection with HERC2 or mock plasmids, the cells were treated with 50 ng/ml IL-6 for 10 min. The localization of the indicated proteins was observed by total internal reflection fluorescence spectroscopy (TIRF). G-H HERC2 knockout (G) and HERC2 overexpression (H) cells were treated with 50 ng/ml IL-6 for 20 min, then membrane protein was isolated and tested for PTP1B levels by western blot analysis. I HEK293T cells were co-transfected with HERC2-Flag and PTP1B mutant-HA plasmids, an immunoprecipitation assay was performed to determine which domain of PTP1B that interacted with HERC2. Data from one representative experiment of three independent experiments are presented