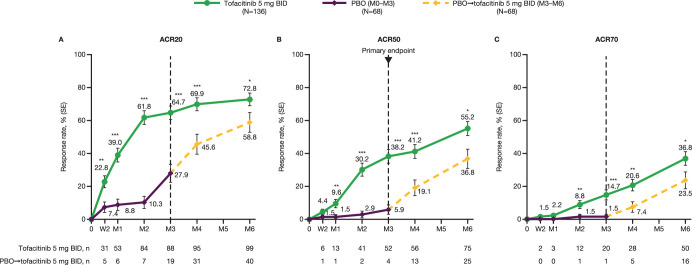
Figure 2.
(A) ACR20, (B) ACR50 and (C) ACR70 response rates to month 6 in Chinese patients with PsA.†‡ The dotted line at month 3 represents the time point at which patients in the placebo group were switched to tofacitinib 5 mg BID from month 3 for the remainder of the study. *p<0.05, **p<0.01, ***p<0.001 versus placebo (through month 3) or placebo→tofacitinib 5 mg BID (for remainder of study). †All randomised patients who received ≥1 dose of study medication. ‡Missing values were considered as non-response. ACR, American College of Rheumatology; ACR20/50/70, ≥20/50/70% improvement, respectively, in ACR response criteria; BID, twice daily; M, month; n, number of patients meeting response criteria; N, number of patients in full analysis set; PBO, placebo; PsA, psoriatic arthritis; SE, standard error; W, week.