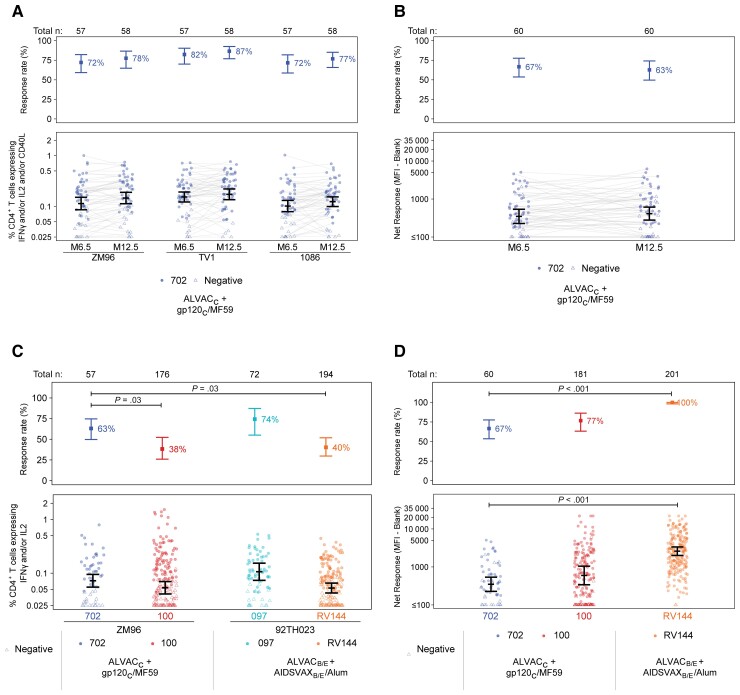
Figure 1.
Characterization of HVTN 702 cellular and humoral immune responses among per-protocol vaccinated noncases and comparison with HVTN 100, HVTN 097, and RV144 per-protocol vaccinated noncases. A, Response rates and magnitudes of CD4+ T cells expressing IFN-γ and/or IL-2 and/or CD40L among HVTN 702 vaccinated noncases, measured by intracellular cytokine staining at months 6.5 and 12.5. B, Month 6.5 and 12.5 IgG binding antibody responses to 1086.C V1V2, HVTN 702. C, Response rates and magnitudes of CD4+ T cells expressing IFN-γ and/or IL-2 among HVTN 702 vaccinated noncases compared to those in HVTN 100, HVTN 097, and RV144 at month 6.5, measured by intracellular cytokine staining. D, Month 6.5 IgG binding antibody responses to 1086.C V1V2 in HVTN 702 compared to HVTN 100 and RV144 (HVTN 097 data not available). Positive response rates and 95% CIs in the top panels and mean magnitudes and 95% CIs in the bottom panels are estimated by targeted maximum likelihood estimation. All Holm-adjusted P values < .05 for HVTN 702 contrasts with earlier trials are displayed. Abbreviations: CI, confidence interval; HVTN, HIV Vaccine Trials Network; IFN-γ, interferon-γ; IgG, immunoglobulin G; IL-2, interleukin 2; MFI, mean fluorescence intensity.