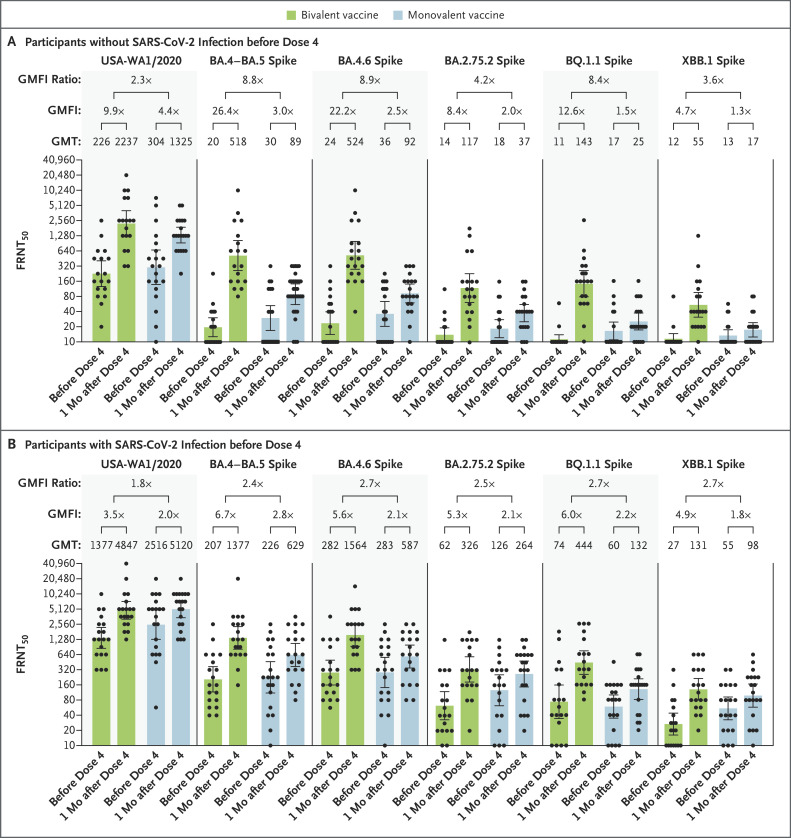
Figure 1. Neutralizing Responses with Bivalent BA.4–BA.5 Vaccine or Monovalent BNT162b2 Booster Vaccine.
Shown is the neutralizing activity against the USA-WA1/2020 strain of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the omicron sublineages BA.4–BA.5, BA.4.6, BA.2.75.2, BQ.1.1, and XBB.1 in all participants who did not have evidence of SARS-CoV-2 infection before the fourth dose of vaccine (Panel A) and in those who had evidence of SARS-CoV-2 infection before the fourth dose of vaccine (Panel B). The heights of the bars and the numbers immediately above the bars indicate the geometric mean titers (GMTs) of neutralizing antibodies. The 𝙸 bars indicate 95% confidence intervals. The results of the fluorescence-based focus reduction neutralization test (FRNT50 [the reciprocal dilution of serum that neutralizes 50% of the input virus]) against USA-WA1/2020, BA.4–BA.5 spike, BA.4.6 spike, BA.2.75.2 spike, BQ.1.1 spike, and XBB.1 spike are shown. Each circle in the figure represents an individual participant. The geometric mean factor increases (GMFI [the increase in neutralizing titers in serum samples from the day of the fourth dose to 1 month after the fourth dose]) and GMFI ratios between GMFIs of bivalent vaccine and GMFIs of monovalent vaccine are shown. In both panels, the lower boundaries of the two-sided 95% confidence intervals for the GMFI of bivalent or monovalent booster against USA-WA1/2020, BA.4–BA.5, BA.4.6, BA.2.75.2, BQ.1.1, and XBB.1 are all greater than 1.