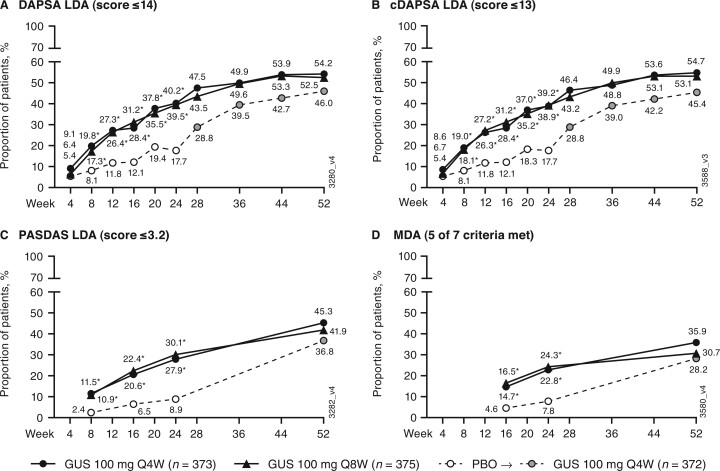
Fig. 1.
Achievement of DAPSA LDA, cDAPSA LDA, PASDAS LDA, and MDA
(A) DAPSA LDA; (B) cDAPSA LDA; (C); PASDAS LDA; (D) MDA. Through week 24, patients meeting treatment failure criteria or with missing data were considered non-responders. Treatment group comparisons through week 24 were not adjusted for multiplicity of testing. After week 24 and through week 52, patients with missing data were considered non-responders. *P < 0.001, vs PBO (nominal). cDAPSA: clinical DAPSA (excludes CRP); DAPSA: Disease Activity in Psoriatic Arthritis; GUS: guselkumab; LDA: low disease activity; MDA: minimal disease activity; PASDAS: Psoriatic Arthritis Disease Activity Score; PBO: placebo; Q4W: every 4 weeks; Q8W: every 8 weeks.