Abstract
Objective
To investigate changes in health-related quality of life (HRQoL) in children and young people with JIA (Juvenile Idiopathic Arthritis) over 3 years following diagnosis.
Methods
Data on children and young people recruited to the Childhood Arthritis Prospective Study (CAPS) were selected if >5 years of age at diagnosis. HRQoL was assessed at diagnosis (baseline), 1 year and 3 years using the proxy-reported Child Health Questionnaire (CHQ) completed by a parent or guardian. The CHQ measures aspects of HRQoL including physical functioning and mental health. Analyses included descriptive statistics, comparison with a US reference population and analysis of CHQ scores longitudinally and by gender and age of onset.
Results
Using CHQ data from parents/guardians of 182 CAPS study participants [median age 9.6 years (interquartile range 7.2–12.2)], all HRQoL domains significantly improved over the 3 year follow-up, except general health perceptions. Physical health domains showed greater improvement than psychosocial domains, although psychosocial scores were generally higher than physical scores throughout. Although similar at diagnosis, at 1 year females had significantly worse HRQoL than males in physical functioning (P = 0.03), bodily pain (P = 0.03), mental health (P = 0.00), social-emotional (P = 0.02) and social-physical (P < 0.001). Differences largely remained at 3 years. Age at onset was not significantly associated with HRQoL.
Conclusion
Children and young people with JIA have low HRQoL across domains compared with the reference population. This improves within 3 years of diagnosis, with the greatest improvement within the first year. Early developmentally appropriate clinical intervention is recommended to reduce both psychosocial and physical impact of JIA. The lower HRQoL scores of females require further investigation.
Keywords: juvenile idiopathic arthritis, health-related quality of life (HRQoL), longitudinal
Rheumatology key messages.
JIA has a substantial impact on parent-reported HRQoL, improving significantly after diagnosis to 3 years.
Although initially higher, improvements in psychosocial components of HRQoL were smaller compared with physical aspects.
Further research should investigate mechanisms for lower HRQoL in females compared with males with JIA.
Introduction
Health-related quality of life (HRQoL) encompasses physical, psychological, social, emotional and behavioural aspects of functioning and well-being [1], domains that have been found to be impacted in children and young people with JIA (Juvenile Idiopathic Arthritis) [2, 3]. The main symptoms of JIA include musculoskeletal pain, stiffness, cognitive and physical fatigue and psychological distress, with possible outcomes including emotional impact and depressive symptoms [4, 5]. The most established classification system to distinguish the disease subtypes was produced by the ILAR (International League of Associationsfor Rheumatology) [6]. It comprises seven subtypes based on clinical signs, symptoms and biochemical laboratory tests. To date, little attention has been given to characterizing which particular domains of HRQoL are affected the most and which remain most affected over time. The physical limitations of JIA have associated complications causing considerable restrictions in daily activities. JIA is likely to affect HRQoL directly due to the nature and physical features of the condition itself and also indirectly due to treatment and impacts on other self-care activities. JIA can also affect aspects of the HRQoL of the wider family/caregiver group as well as the individual [7].
In addition to the direct impact of living daily with JIA, there are also several treatment-related issues that may impact upon HRQoL. For example, delays in seeking or gaining access to effective therapies are a common experience and often detrimental to HRQoL [8]. Continued uncertainty about which treatments provide the best outcomes for which individual means that ‘trial and error’ approaches frequently contribute to these delays. This further complicates our understanding of both physical and psychological outcomes, impacts and prognoses and what treatment(s) to prescribe [9]. Studies of the long-term outcome in children and young people with JIA have found that a substantial proportion do not achieve clinical remission despite current therapies [10, 11]. Some treatment options or regimens are more burdensome than others when taking account of side effects or painful administration procedures such as intra-articular corticosteroid injections [12]. Non-pharmacological options including psychological, occupational and physical therapies require considerable time commitments for those with JIA and their families.
Psychological functioning (such as regulation of mood, coping responses) in JIA is a key component of HRQoL. Outcomes of interest include long-term effects of JIA on mood, self-esteem, social functioning and family relationships. A systematic review by Fair et al. [3] found that much of the existing literature consists of cross-sectional studies. Although it is clear that these types of studies are needed, new prioritized research agendas for rheumatology research have concluded that large-scale, multicentre studies will enable greater investigation of disparities of psychological outcomes, particularly in children and young people [13, 14]. Multicentre studies help to address the issue of insufficient statistical power in rare diseases by pooling patient numbers.
Existing longitudinal studies of HRQoL in JIA have reported improvement in overall HRQoL after diagnosis, with different levels of improvement for psychological and physical aspects over time [15–17]. It is unclear whether specific domains within these scores change at different rates and so may require earlier intervention.
Research focused on characterizing the relationship between JIA and HRQoL is needed, particularly exploration of the associations over the longer-term disease course and exploring the role of individual and disease characteristics upon HRQoL in this group [13, 14]. The current study aims to address this gap in the literature by reporting HRQoL domains from the point of diagnosis and whether this changes within 3 years following a JIA diagnosis. A 3 year time point was chosen to show the outcome of JIA in the years following diagnosis, particularly since the ‘window of opportunity’ for optimal treatment response is roughly 6 months–1 year, so this study shows both short- and longer-term outcomes. A secondary aim was to look at the relationship of additional factors, including gender, for which the lower HRQoL of females merits further investigation [18, 19], and age at onset on HRQoL.
Methods
Study design
The Childhood Arthritis Prospective Study (CAPS) is a multicentre prospective inception cohort study established in the UK in 2001. The purpose of CAPS is to investigate the environmental, clinical and genetic factors that predict and explain both short- and long-term outcomes in JIA [20]. Children and young people with newly diagnosed inflammatory arthritis in one or more joints persisting for at least 2 weeks were invited to participate in CAPS; however, for the current study, analysis was limited to participants with a subsequent diagnosis of JIA, in keeping with ILAR criteria for JIA. Study exclusion criteria were septic arthritis, haemarthrosis, arthritis due to malignancy or trauma and CTD. All were <16 years of age at onset and had presented to paediatric rheumatology outpatient clinics (for which the first visit became the baseline) or were admitted as inpatients. Additional cohort details have been described elsewhere [21]. CAPS has recruited participants from seven UK paediatric rheumatology centres.
The study received ethical approval from the UK Northwest Multicentre Research Ethics Committee (REC 02/8/104) [8] and complies with the Declaration of Helsinki. Parents/guardians of participants provided informed written consent and children were asked to provide assent where appropriate [20].
Data collection
CAPS has two main streams of data collection, one being patient-reported outcome measures completed by participants and their families and the other being clinical and demographic data from medical records provided by the UK paediatric rheumatology centres, including gender, age at onset, JIA subtype, Child Health Assessment Questionnaire (CHAQ), Parent Global Assessment of their child’s health [visual analogue scale (VAS)], Physician Global Assessment of health [visual analogue scale (VAS)], active joint count, limited joint count and ESR [20, 21].
Age of onset was collected at baseline and dichotomized into 5–10 years and ≥11 years for the current study to determine what effect an adolescent onset had, if any.
HRQoL assessment
HRQoL was assessed using the 50-item version of the Child Health Questionnaire (CHQ) [22]. The CHQ was developed to measure HRQoL in children and young people ≥5 years of age [22] in a similar manner to the adult 36-item Short-Form Health Survey [23]. The CHQ is a generic HRQoL instrument that has demonstrated good internal consistency and construct validity as well as appropriate sensitivity to changes in disease activity [24].
The CHQ was designed to be completed by one of the parents or guardians of the study participants. Although there are limits to proxy reporting, in this case it provides consistency of reporting and overcomes difficulties of data collection in very young children. The questionnaire assesses 15 health domains, with 11 domains relating to the person directly (physical functioning, bodily pain/discomfort, global health, general health perceptions, social functioning-physical, social functioning-emotional/behavioural, change in health, behaviour, mental health, self-esteem and global behaviour) and the remaining four domains relating to the impact on parents and the wider family (parent impact-time, parent impact-emotional, family activities and family cohesion) [25]. This analysis aimed to understand the data for the first 11 domains, namely the effects of JIA on the children’s and young peoples’ HRQoL.
The parent/guardian completes the CHQ by recalling the preceding 4 weeks, except for the subscale change in health, for which the recall period is 1 year, and the general health perceptions and global health subscales, which enquire about the child’s health in general [24]. Each item contains four to six response options that are transformed into a 0–100 scale, for which a lower score represents a poorer health state. In addition, the individual subscale scores have been aggregated to form two summary measures: the physical summary and psychosocial summary scores. For the physical summary and psychosocial summary scores, the mean norm score is 50 (s.d. 10). Lower summary scores suggest poorer HRQoL [24]. All domains contribute to both summary measures, although coefficients differ depending on relevance to the summary measure in question.
Data from a representative sample (N = 391, of which 212 were male and 177 female) of the general US population, ages 5–18 years and collected in 1994 [22], was also included for comparison with the CAPS cohort (Tables 1 and 2).
Table 1.
Characteristics of study populations
| Characteristics | CAPS (n = 184) | US population sample (n = 391) | |
|---|---|---|---|
| Gender, n (%) | Male | 74 (41) | 212 (54) |
| Female | 108 (59) | 177 (46 | |
| Age (males and females, CAPS age at baseline), years, n (%) | 5–7 | 57 (31) | 73 (19%) |
| 8–10 | 57 (31) | 77 (20) | |
| 11–12 | 36 (20) | 83 (21) | |
| 13–15 | 30 (17) | 96 (25) | |
| 16–18 | 2 (1) | 63 (16) | |
Table 2.
Mean and median CHQ scores at baseline, 1 year and 3 years post-diagnosis
| CHQ domain | Population sample (N = 391), mean (s.d.) | CAPS baseline score |
CAPS 1 year |
CAPS 3 years |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N (%)a | Mean (s.d.) | Median (IQR) | N (%)a | Mean (s.d.) | Median (IQR) | N (%)a | Mean (s.d.) | Median (IQR) | ||
| Physical functioning | 96.1 (13.9) | 182 (100) | 64 (32.6) | 72.2 (38.9–94.4) | 181 (99) | 79.8 (26.1) | 88.9 (66.7–100) | 180 (99) | 77.9 (29.4) | 94.4 (61.1–100) |
| Bodily pain | 81.7 (19) | 171 (94) | 47.6 (29.2) | 50 (20–70) | 181 (99) | 63.4 (26.8) | 60 (40–80) | 180 (99) | 64.5 (29.6) | 70 (40–100) |
| Change in health | NA | 180 (99) | 2.3 (1.1) | 2 (2–3) | 178 (97) | 3.8 (1) | 4 (3–5) | 179 (98) | 3.5 (1.1) | 3 (3–5) |
| General health perceptions | 73 (17.3) | 181 (99) | 61.3 (19.8) | 64.2 (46.7–76.7) | 179 (98) | 57.6 (20.1) | 60 (43.3–72.5) | 181 (99) | 56.5 (21.5) | 60 (39.2–72.5) |
| Behaviour | 75.6 (16.7) | 170 (93) | 68.8 (20.3) | 72.5 (55.8–83.3) | 181 (99) | 73 (19) | 76.7 (60–89.2) | 181 (99) | 73.6 (20) | 76.7 (68.3–87.5) |
| Mental health | 78.5 (13.2) | 171 (94) | 70 (20.1) | 75 (60–85) | 181 (99) | 77.8 (16.3) | 80 (70–90) | 181 (99) | 77 (17.7) | 80 (65–90) |
| Self-esteem | 79.8 (17.5) | 170 (93) | 68.4 (22.1) | 70.8 (54.2–87.5) | 181 (99) | 74.6 (19.6) | 75 (62.5–91.7) | 181 (99) | 72.8 (21.4) | 75 (58.3–87.5) |
| Social-emotional | 92.5 (18.6) | 181 (99) | 73.5 (34.5) | 100 (44.4–100) | 179 (98) | 85 (26.5) | 100 (77.8–100) | 181 (99) | 81.8 (29.7) | 100 (66.7–100) |
| Social-physical | 93.6 (18.6) | 182 (100) | 61.1 (37.4) | 66.7 (33.3–100) | 179 (98) | 79.7 (29.2) | 100 (66.7–100) | 180 (99) | 79.6 (31.9) | 100 (66.7–100) |
| Physical summary score | 53 (8.8) | 169 (93) | 33.8 (17.7) | 37.1 (19.8–49) | 175 (96) | 42.1 (15) | 47.5 (34.4–53.3) | 176 (97) | 41.8 (17.1) | 48.6 (33–54.6) |
| Psychosocial summary score | 51.2 (9.1) | 169 (93) | 46.2 (12.1) | 49.4 (37.8–55.2) | 175 (96) | 50.6 (10.4) | 52.1 (44.9–58.8) | 176 (97) | 50.4 (10.9) | 53.8 (45.7–58) |
NA, not available.
% complete.
Analysis
Statistical analysis of the dataset was performed using Stata/IC version 14 (StataCorp, College Station, TX, USA). Domain scores were compared between baseline and 1 year, 1 year and 3 years and baseline and 3 years and also with those from the population sample of US children (available at a single time point) using descriptive statistics. For additional longitudinal analysis, differences between CAPS median CHQ domain scores at baseline and 3 years were assessed using the Wilcoxon signed-rank test. At baseline, 1 year and 3 years, differences between the scores of female and male participants and differences between the two categorized ages of onset were assessed using the Mann–Whitney U test. All analyses were complete case, in that participants were included if parents had completed the CHQ at each of the three assessment points.
Results
Study population
For the current analysis, data were included if the CAPS study participants were >5 years of age at diagnosis (minimum age as recommended by CHQ guidelines [22]) and parents had reported data for all domains of the CHQ from each of the three assessment points—baseline (diagnosis), 1 year post-diagnosis and 3 years post-diagnosis.
Of the 1503 CAPS participants recruited by 2015 [22], parents/guardians of 182 participants had completed CHQs at the above three time points. These participants were recruited between 2001 and 2012.
Demographic details for the 182 children and young people whose data were included in the current study and CAPS participants who were not included (less than three CHQs completed) are broadly similar in terms of gender and ILAR distribution (Table 3). There were no differences between those included and excluded in terms of CHAQ score, ESR, active and limited joint count, parent VAS, physician VAS and gender using Mann–Whitney U-tests. However, those included had a lower median age at onset {8.8 years [interquartile range (IQR) 6.6–11.1]} than those excluded [10.2 years (IQR 7–12.5), P = 0.001].
Table 3.
Demographic data for CAPS participants included and excluded from the current study
| Characteristics | Included (n = 182) | Included, %/median (IQR) | Excluded (n = 820) | Excluded, %/median (IQR) | |
|---|---|---|---|---|---|
| Gender | Male | 74 | 41 | 345 | 42 |
| Female | 108 | 59 | 475 | 58 | |
| Age at onset, years | 5–10 | 101 | 55 | 306 | 37 |
| ≥11 | 40 | 22 | 250 | 31 | |
| Missing or <5 | 41 | 22 | 264 | 32 | |
| Overall | 153 | 8.8 (6.6–11.1) | 602 | 10.2 (7–12.5) | |
| JIA subtype | Systemic arthritis | 8 | 4 | 55 | 7 |
| Persistent oligoarthritis | 75 | 41 | 292 | 36 | |
| Extended oligoarthritis | 7 | 4 | 17 | 2 | |
| Polyarthritis (RF−) | 27 | 15 | 131 | 16 | |
| Polyarthritis (RF+) | 6 | 3 | 31 | 4 | |
| ERA | 12 | 7 | 58 | 7 | |
| Psoriatic arthritis | 15 | 8 | 68 | 8 | |
| Unclassifiable JIA | 32 | 18 | 110 | 13 | |
| Other inflammatory arthritis | 0 | 0 | 30 | 4 | |
| ILAR missing | 0 | 0% | 26 | 3% | |
| Core outcome variables | CHAQ | 164 | 0.625 (0.125–1.375) | 451 | 0.625 (0.125–1.25) |
| Parent VAS | 164 | 16.5 (4–49) | 409 | 24 (5–50) | |
| Physician VAS | 144 | 30 (16–53) | 502 | 28 (15–49) | |
| Active joint count | 165 | 2 (1–6) | 611 | 2 (1–5) | |
| Limited joint count | 165 | 1 (1–4) | 611 | 1 (0–4) | |
| ESR | 101 | 19 (7–44) | 464 | 20 (7–50) | |
The majority of children and young people whose date were included were female (59%) and the most common ILAR category was persistent oligoarthritis (41%). At diagnosis, the median number of active joints was 2 (IQR 1–6) with 1 limited joint (IQR 1–4) (Table 3).
HRQoL over time
Table 2 presents descriptive data for each CHQ domain at baseline, 1 year and 3 years post-diagnosis. The mean CHQ scores at baseline were lower compared with the national norms (also parent-proxy reports) for the population sample of US children [22]. The largest differences were in the physical functioning (CAPS score 64 vs US sample 96.1), bodily pain (47.6 vs 81.7) and social-physical (61.1 vs 93.6) domains.
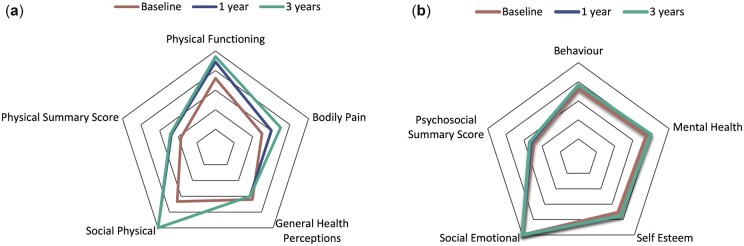
The majority of CHQ domain scores improved following baseline. The largest change occurred from baseline to 1 year post-diagnosis (Fig. 1A and B). The exception to this was the domain general health perceptions, for which the mean CHQ score decreased after baseline. There was a greater improvement over time for the physical health domains (i.e. physical functioning baseline median 72.2 vs 94.4 at 3 years, P = <0.001; bodily pain baseline median 50 vs 70 at 3 years, P = <0.001) than the psychological and social domains (i.e. mental health baseline median 75 vs 80 at 3 years, P = <0.001; self-esteem baseline median 70.8 vs 75 at 3 years, P = 0.01), although scores for the latter two areas were higher from the outset (Table 2). Statistically significant differences (P < 0.05) between CAPS baseline and 3 year scores were found for all 11 domains.
Fig. 1.
CHQ domain scores: physical and psychosocial
(A) Median CHQ scores for physical aspects of HRQoL at baseline, 1 year and 3 years post-diagnosis. (B) Median CHQ scores for psychosocial aspects of HRQoL at baseline, 1 year and 3 years post-diagnosis.
By 3 years following JIA diagnosis, CAPS participants’ scores had improved (except general health perceptions) but continued to be worse than the comparison group across all domains.
However, by the 3 year time point, scores were similar between the two groups for the behaviour (CAPS score 73.6 vs US sample 75.6) and mental health (77.1 vs 78.5) domains plus the psychosocial summary score (50.4 vs 51.2). CHQ scores from the population sample were not available for the change in health domain.
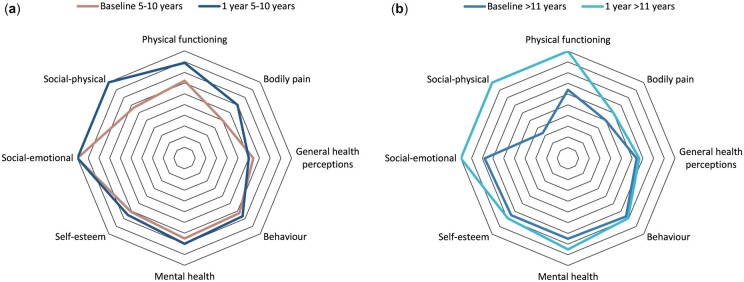
HRQoL and age of onset
There were no statistically significant differences in any HRQoL domain scores between the two age at onset groups at any of the three time points (Supplementary Tables S1–S3, available at Rheumatology online). All domain scores for both groups increased from baseline to 1 year (Fig. 2A and B), except general health perceptions in the 5–10 years onset group. Domain scores at 3 years were similar between the age at onset groups.
Fig. 2.
CHQ domain scores: age at onset
(A) Median CHQ scores for ages 5–10 years at onset (baseline and 1 year post-diagnosis; year 3 data not presented as similar to year 1). (B) Median CHQ scores ages >11 years at onset (baseline and 1 year post-diagnosis; year 3 data not presented as similar to year 1).
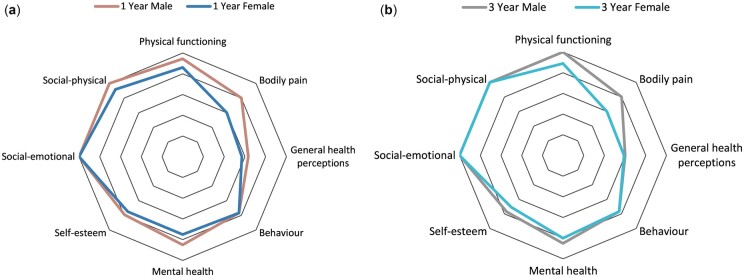
HRQoL and gender
There were no significant differences in CHQ scores between genders at baseline (Supplementary Table S4, available at Rheumatology online). All scores increased after baseline, although there was a more pronounced increase in scores for male participants at 1 year and 3 years (Supplementary Tables S5 and S6, available at Rheumatology online), with males scoring higher in every domain except behaviour [female 3 year median 76.7 (IQR 68.3–87.5), male 3 year median 76.7 (IQR 64.2–89.2), P = 0.8803] at both these time points (Fig. 3A and B). At 1 year, females had lower scores than males across physical functioning (P = 0.0244), bodily pain (P = 0.0177), mental health (P = 0.0031), role/social-emotional (P = 0.0240) and role/social-physical (P = 0.0028). These differences remained at 3 years, with the exception of role/social-emotional (P = 0.0667).
Fig. 3.
CHQ domain scores: gender
(A) Median CHQ scores by gender (1 year post-diagnosis). (B) Median CHQ scores by gender (3 years post-diagnosis).
Importantly, there were no differences between males and females in terms of ILAR (P = 0.7549), CHAQ score (P = 0.4843), parent VAS (P = 0.5286), active joints (P = 0.4089), limited joints (P = 0.1867) and ESR (P = 0.7216) using Mann–Whitney U-tests (Supplementary Table S7, available at Rheumatology online) and physician VAS scores for males were lower than for females (P = 0.0409).
Discussion
To our knowledge this is one of few studies [15–17] presenting longitudinal data showing the impact of JIA on the different facets of HRQoL and how this changes over time.
Furthermore, with the exception of Listing et al. [16], previous longitudinal HRQoL studies combined both children and young people and proxy-reported (usually parent-reported) data. While there are limitations with parents or other proxies reporting on QoL, an approach that collates longitudinal data consistently from the same source overcomes the significant problem of switching data sources from parent to child reports.
The results indicated lower HRQoL, both physical and psychosocial, than for the reference population of US children [22] at diagnosis, and despite gradual improvement over time, this remained lower throughout the 3 years of data analysed. However, the US sample had a more even age distribution than the CAPS cohort, the latter having a higher proportion in the younger age ranges (Table 1). This could be because participants were recruited to CAPS at a younger age, i.e. typically when first diagnosed. The US sample also had a greater distribution of males vs females [54% male as opposed to 41% in CAPS (Table 1)], which could be explained by a higher prevalence of JIA in females [26].
The largest improvement in the CAPS cohort occurred from diagnosis to 1 year post-diagnosis, a time that would likely coincide with initiation of therapies and is similar to the findings of Listing et al. [16] and Oen et al. [15]. Listing et al. [16] also found that physical health was more affected by JIA than psychosocial health and there is some correlation with our data in that psychosocial scores were generally higher than physical scores throughout the study, although the latter did not improve at the same rate as physical scores.
The domains that were most affected were bodily pain, physical functioning, general health perceptions, role/social-emotional and role/social-physical. The impact on physical facets of HRQoL was to be expected due to the substantial physical limitations and pain caused by the affected joints, however, a significant impact on the social aspects of HRQoL, from both emotional and physical difficulties, was also reported, indicating that JIA is substantially affecting life at school and activities with friends.
Non-physical aspects have been shown to have a sizeable impact on HRQoL and HRQoL can be adversely affected even when clinical symptoms are minimal [27]. Fears around social acceptance and uncertainty about the future may contribute to the lack of improvement in psychosocial scores, although further research is needed. Certainly findings from the current study further highlight the need to address the specific psychological needs of young people with JIA [14].
Although there was a general increase in HRQoL over time for most aspects of QoL assessed in the CHQ, the domain general health perceptions worsened after diagnosis. This could be related to the psychosocial impact of drug treatment given that treatment itself (such as DMARDs and biologic therapies) can have side effects that can adversely affect HRQoL [28, 29]. Another possible explanation is that children and young people and their families may view JIA initially as a short-term illness, but this view may change as they learn more about it. Knowledge of the potential short- and long-term outcomes for their child is very important to parents [30]. This may lead to a shift in perception towards having a child with a long-term condition, with the implications of this realization increasing over the first few years following diagnosis. Since the relapsing–remitting nature of the condition is not immediately apparent, there may have been uncertainty about disease chronicity at the point of diagnosis [31, 32].
Although there were no statistically significant differences between the two age-at-onset groups, the adolescent group reported worse HRQoL in the domains physical functioning and particularly role/social-emotional and role/social-physical at baseline. These results suggest that the detrimental effects of JIA on social functioning are more acute for adolescents than they are for younger children at diagnosis, although this does improve following treatment. This is not surprising when one considers the potential impact of conditions like JIA on adolescent social and emotional development if disease onset occurs during adolescence [33, 34].
Since this study focused on the parental perspective of the child’s HRQoL, consideration of the impact of JIA on parents should also be considered in the interpretation of the data. Gomez-Ramirez et al. [7] report that parents of children with JIA describe a complex journey of recurrent negative and positive emotions, as opposed to a linear progression towards a positive resolution. This may help to explain the lack of improvement in the general health perceptions domain in the current study and that HRQoL may fluctuate if we observe over a longer period, as opposed to continuing to improve [17].
Our data add to the evidence base that lower HRQoL has been associated with female gender in studies investigating the long-term impact of JIA on HRQoL [18, 19, 35, 36]. Given that these findings appear not to be due to differences in levels of disease severity between males and females, further research is needed into the relationships between mental health, gender and HRQoL during adolescence.
Strengths and limitations
Describing the impact of and improvement in the specific HRQoL domains has allowed us to identify the specific physical and psychosocial aspects of HRQoL that experience the most detrimental effects over time, in contrast to previous cross-sectional studies comparing summary measures.
One feature of the study design that is a possible limitation is proxy reporting of HRQoL. The parental perspective is only one perspective and may be different, particularly during adolescence when young people begin to live more independently. That being said, the parental perspective remains an important consideration both clinically and from a research perspective [30] and, importantly for the current study, it provided crucial consistency in terms of the data informant across the three time periods. Changing the source to the children and young people once they were old enough to self-report could potentially confound results.
We selected this analysis precisely because the CHQ enables inclusion of younger children too (≥5 years old), although we still had to reduce the available cohort by removing CAPS participants who were younger than this. Further prospective research into HRQoL reported by the young people themselves, as is being collected by the REACH study [37], and whether this differs from the parental perspective is awaited with interest. However, a study by Shaw et al. [38] found ∼50% disagreement in HRQoL reports between adolescents and their parents as measured by the Juvenile Arthritis Quality of Life Questionnaire.
Given the lack of a recent UK comparison sample, our only option was to use previously published US norms for a 1994 (non-clinical) community population of US children [22]. As such, we acknowledge cultural and historical differences in HRQoL between the populations.
Although outside the scope of the current study, assessment of the impact of specific drug therapies on HRQoL merits further investigation. Further studies should address the direct effects of therapy on HRQoL via disease control as well as the potential impacts caused by side effects and burden of adherence during adolescent development.
Clinical implications
Our study described the detrimental physical and psychosocial effects of JIA on HRQoL. While HRQoL is reported to be significantly poorer in those with JIA at diagnosis, our study suggests that professionals can reassure children and young people and their families that, in general, there is a significant improvement in HRQoL within the first year following diagnosis and further improvement during the following years.
Furthermore, our results suggest support that addresses psychological and social aspects of the condition is essential for children and young people with JIA and their families, as is advocated by current standards of care [39, 40]. Our results also suggest that females are at greater risk of poorer HRQoL. These areas should be addressed routinely and regularly during consultations to identify any potentially unmet needs.
Conclusions
This study provides a detailed account of the impact of JIA on the different facets of HRQoL and how this changes in the first 3 years following a diagnosis of JIA.
Our results show that HRQoL is still considerably lower than that of the reference population and, despite improvement, continues to be lower at 3 years post-diagnosis for all domains.
Although our results showed that JIA had a detrimental effect on all areas of HRQoL, these results also suggest that HRQoL generally improves within the first year following diagnosis, with further improvement during the following years.
It is essential that in the future, support for individuals with JIA and their families should routinely include monitoring potential effects of disease on both psychological and social functioning as well as physical outcomes. Our results justify a greater focus on providing support to address the psychological and social impact of JIA early in paediatric rheumatology care [41]. This is best provided by a multidisciplinary paediatric and adolescent rheumatology team, ideally including medicine, nursing, physiotherapy, occupational therapy, social work, psychology and school liaisons [14]. As female JIA patients appear to be at greater risk of poorer HRQoL, additional awareness of the needs of this group may be important.
Acknowledgements
We thank all the children and young people and their families who have contributed to CAPS. We thank Versus Arthritis for funding the Special Strategic Grant entitled ‘Childhood Arthritis Prospective Study (CAPS)’ (UK grant 20542). We also thank all principal investigators, clinical staff and research coordinators of the CAPS who have made this research possible, as well as members of the research team at the University of Manchester, UK. These include W. Thomson (University of Manchester, UK), G. Cleary and E. Baildam (Alder Hey Children’s Hospital, UK), L. R. Wedderburn (Great Ormond Street Hospital, UK), J. Davidson (Royal Hospital for Sick Children, Edinburgh and Royal Hospital for Children, Glasgow, UK), A. Chieng (Royal Manchester Children’s Hospital, UK), F. McErlane and H. Foster (Royal Victoria Infirmary, UK), C. Ciurtin and Y. Ioannou (University College London Hospital, UK). The research team at the University of Manchester are additionally supported by the Centre for Epidemiology Versus Arthritis (UK grant 21755) and the Centre for Genetics and Genomics Versus Arthritis (UK grant 21754). A.D.S., R.R.L., S.S.-W., K.L.H., J.E.M. and L.C. are also supported by the NIHR Manchester Biomedical Research Centre. L.R.W. is supported by the NIHR Biomedical Research Centre at Great Ormond Street Hospital. The views expressed herein are those of the authors and not necessarily those of the National Health Service, National Institute for Health Research or UK Department of Health.
All authors were involved in drafting the article or revising it critically for important intellectual content and all authors approved the final version to be submitted for publication. A.D.S. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. A.D.S., B.S.,R.R.L. and L.C. were responsible for study conception and design. A.D.S., K.L.H., J.E.M. and L.C. were responsible for the acquisition of data. All authors were responsible for the analysis and interpretation of data.
Funding: This work was supported by the NIHR Manchester Biomedical Research Centre and Versus Arthritis, including funding for the Centre for Epidemiology Versus Arthritis (grant 20380) and Centre for Genetics and Genomics (grant 20385) and the Special Strategic Grant entitled ‘Childhood Arthritis Prospective Study (CAPS)’ (UK grant 20542).
Disclosure statement: J.E.M. has received advisory board membership fees from Pfizer and CSL-Behring. K.L.H. has received honoraria from AbbVie and grant income from Pfizer and Bristol Myers Squibb. The remaining authors declare no conflicts of interest relating to this work.
Supplementary Material
Contributor Information
Andrew D Smith, Centre for Epidemiology Versus Arthritis, Centre for Musculoskeletal Research, University of Manchester; NIHR Manchester Biomedical Research Centre, Manchester University NHS Foundation Trust, Manchester Academic Health Science Centre.
Bishma Saqib, Centre for Epidemiology Versus Arthritis, Centre for Musculoskeletal Research, University of Manchester; Centre for Genetics and Genomics Versus Arthritis, Centre for Musculoskeletal Research.
Rebecca Rachael Lee, Centre for Epidemiology Versus Arthritis, Centre for Musculoskeletal Research, University of Manchester; NIHR Manchester Biomedical Research Centre, Manchester University NHS Foundation Trust, Manchester Academic Health Science Centre.
Stephanie Shoop-Worrall, Centre for Epidemiology Versus Arthritis, Centre for Musculoskeletal Research, University of Manchester; Centre for Health Informatics, University of Manchester.
Kimme L Hyrich, Centre for Epidemiology Versus Arthritis, Centre for Musculoskeletal Research, University of Manchester; NIHR Manchester Biomedical Research Centre, Manchester University NHS Foundation Trust, Manchester Academic Health Science Centre.
Janet E McDonagh, Centre for Epidemiology Versus Arthritis, Centre for Musculoskeletal Research, University of Manchester; NIHR Manchester Biomedical Research Centre, Manchester University NHS Foundation Trust, Manchester Academic Health Science Centre; Department of Paediatric and Adolescent Rheumatology, Royal Manchester Children’s Hospital, Manchester University Hospitals Trust, Manchester, UK.
Lis Cordingley, Centre for Epidemiology Versus Arthritis, Centre for Musculoskeletal Research, University of Manchester; NIHR Manchester Biomedical Research Centre, Manchester University NHS Foundation Trust, Manchester Academic Health Science Centre.
Data availability statement
The data underlying this article will be shared upon reasonable request to the corresponding author.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Stevanovic D, Susic G.. Health-related quality of life and emotional problems in juvenile idiopathic arthritis. Qual Life Res 2013;22:607–12. [DOI] [PubMed] [Google Scholar]
- 2. Sawyer MG, Whitham JN, Roberton DM. et al. The relationship between health-related quality of life, pain and coping strategies in juvenile idiopathic arthritis. Rheumatology (Oxford) 2004;43:325–30. [DOI] [PubMed] [Google Scholar]
- 3. Fair DC, Rodriguez M, Knight AM, Rubinstein TB.. Depression and anxiety in patients with juvenile idiopathic arthritis: current insights and impact on quality of life, a systematic review. Open Access Rheumatol 2019;11:237–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hanns L, Cordingley L, Galloway J. et al. Depressive symptoms, pain and disability for adolescent patients with juvenile idiopathic arthritis: results from the Childhood Arthritis Prospective Study. Rheumatology (Oxford) 2018;57:1381–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Margetić B, Aukst-Margetić B, Bilić E, Jelusić M, Tambić Bukovac L.. Depression, anxiety and pain in children with juvenile idiopathic arthritis (JIA). Eur Psychiatry 2005;20:274–6. [DOI] [PubMed] [Google Scholar]
- 6. Petty RE, Southwood TR, Manners P. et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol 2004;31:390–2. [PubMed] [Google Scholar]
- 7. Gómez-Ramírez O, Gibbon M, Berard R. et al. A recurring rollercoaster ride: a qualitative study of the emotional experiences of parents of children with juvenile idiopathic arthritis. Pediatr Rheumatol 2016;14:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McErlane F, Foster HE, Carrasco R. et al. Trends in paediatric rheumatology referral times and disease activity indices over a ten-year period among children and young people with juvenile idiopathic arthritis: results from the childhood arthritis prospective Study. Rheumatology (Oxford) 2016;55:1225–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Foster HE, Eltringham MS, Kay LJ. et al. Delay in access to appropriate care for children presenting with musculoskeletal symptoms and ultimately diagnosed with juvenile idiopathic arthritis. Arthritis Care Res 2007;57:921–7. [DOI] [PubMed] [Google Scholar]
- 10. Glerup M, Herlin T, Twilt M.. Clinical outcome and long-term remission in JIA. Curr Rheumatol Rep 2017;19:1–11. [DOI] [PubMed] [Google Scholar]
- 11. Glerup M, Rypdal V, Arnstad ED. et al. Long-term outcomes in juvenile idiopathic arthritis: eighteen years of follow-up in the population-based Nordic juvenile idiopathic arthritis cohort. Arthritis Care Res 2020;72:507–16. [DOI] [PubMed] [Google Scholar]
- 12. Oren-Ziv A, Hoppenstein D, Shles A, Uziel Y.. Sedation methods for intra-articular corticosteroid injections in juvenile idiopathic arthritis: a review. Pediatr Rheumatol 2015;13:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rubinstein TB, Ogbu EA, Rodriguez M. et al. Prioritized agenda for mental health research in pediatric rheumatology from the childhood arthritis and rheumatology research alliance mental health workgroup. J Rheumatol 2020;47:1687–95. [DOI] [PubMed] [Google Scholar]
- 14. Cordingley L, Lee RR.. Can we implement the new research agenda for mental health? Nat Rev Rheumatol 2020;16:191–2. [DOI] [PubMed] [Google Scholar]
- 15. Oen K, Guzman J, Dufault B. et al. Health-related quality of life in an inception cohort of children with juvenile idiopathic arthritis: a longitudinal analysis. Arthritis Care Res 2018;70:134–44. [DOI] [PubMed] [Google Scholar]
- 16. Listing M, Mönkemöller K, Liedmann I. et al. The majority of patients with newly diagnosed juvenile idiopathic arthritis achieve a health-related quality of life that is similar to that of healthy peers: results of the German multicenter inception cohort (ICON). Arthritis Res Ther 2018;20:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tollisen A, Selvaag AM, Aasland A, Lerdal A, Flatø B.. Longitudinal health status from early disease to adulthood and associated prognostic factors in juvenile idiopathic arthritis. J Rheumatol 2019;46:1335–44. [DOI] [PubMed] [Google Scholar]
- 18. Kyllönen MS, Ebeling H, Kautiainen H, Puolakka K, Vähäsalo P.. Psychiatric disorders in incident patients with juvenile idiopathic arthritis – a case–control cohort study. Pediatr Rheumatol 2021;19:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hankin BL, Young JF, Abela JRZ et al. Depression from childhood into late adolescence: Influence of gender, development, genetic susceptibility, and peer stress. J Abnorm Psychol 2015;124:803–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Adib N, Hyrich K, Thornton J. et al. Association between duration of symptoms and severity of disease at first presentation to paediatric rheumatology: results from the Childhood Arthritis Prospective Study. Rheumatology (Oxford) 2008;47:991–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hyrich KL, Lal SD, Foster HE. et al. Disease activity and disability in children with juvenile idiopathic arthritis one year following presentation to paediatric rheumatology. Results from the Childhood Arthritis Prospective Study. Rheumatology (Oxford) 2010;49:116–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Landgraf JM, Abetz LN, Ware JE. The CHQ scoring and interpretation manual. Cambridge, MA: HealthActCHQ, 2008.
- 23. Ware JE, Sherbourne CD.. The MOS 36-item short-form health survey (SF-36): I. conceptual framework and item selection. Med Care 1992;30:473–83. [PubMed] [Google Scholar]
- 24. Hullmann SE, Ryan JL, Ramsey RR, Chaney JM, Mullins LL.. Measures of general pediatric quality of life: Child Health Questionnaire (CHQ), DISABKIDS Chronic Generic Measure (DCGM), KINDL-R, Pediatric Quality of Life Inventory (PedsQL) 4.0 Generic Core Scales, and Quality of My Life Questionnaire (QoML). Arthritis Care Res 2011;63(Suppl 11):420–30. [DOI] [PubMed] [Google Scholar]
- 25. Gutiérrez-Suárez R, Pistorio A, Cespedes Cruz A. et al. Health-related quality of life of patients with juvenile idiopathic arthritis coming from 3 different geographic areas. The PRINTO multinational quality of life cohort study. Rheumatology (Oxford) 2006;46:314–20. [DOI] [PubMed] [Google Scholar]
- 26. Thierry S, Fautrel B, Lemelle I, Guillemin F.. Prevalence and incidence of juvenile idiopathic arthritis: a systematic review. Joint Bone Spine 2014;81:112–7. [DOI] [PubMed] [Google Scholar]
- 27. Palman J, Shoop-Worrall S, Hyrich K, McDonagh JE.. Update on the epidemiology, risk factors and disease outcomes of juvenile idiopathic arthritis. Best Pract Res Clin Rheumatol 2018;32:206–22. [DOI] [PubMed] [Google Scholar]
- 28. Kearsley-Fleet L, Gonzá Lez LV, Steinke D. et al. Methotrexate persistence and adverse drug reactions in patients with juvenile idiopathic arthritis. Rheumatology (Oxford) 2019;58:1453–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Klein A, Becker I, Minden K. et al. Biologic therapies in polyarticular juvenile idiopathic arthritis. comparison of long‐term safety data from the German BIKER Registry. ACR Open Rheumatol 2020;2:37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wright J, Curran J, Rose-Davis B. et al. Parental perspectives about research and knowledge translation in juvenile idiopathic arthritis. ACR Open Rheumatol 2020;2:138–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tong A, Jones J, Craig JC, Singh-Grewal D.. Children’s experiences of living with juvenile idiopathic arthritis: a thematic synthesis of qualitative studies. Arthritis Care Res (Hoboken) 2012;64:1392–404. [DOI] [PubMed] [Google Scholar]
- 32. Waite-Jones JM, Swallow V, Madill A.. From ‘neurotic’ to ‘managing’ mother: the ‘medical career’ experienced by mothers of a child diagnosed with juvenile idiopathic arthritis. Br J Health Psychol 2020;25:324–38. [DOI] [PubMed] [Google Scholar]
- 33. Cai RA, Chaplin H. . Impact of rheumatic musculoskeletal disease on psychological development in adolescents and young adults. In: McDonagh J, Tattersall R, eds. Adolescent and young adult rheumatology in clinical practice. Cham: Springer, 2019:19–33. [Google Scholar]
- 34. Chaplin H, Cai A. . Impact of rheumatic disease on social development in adolescents and young adults. In: McDonagh J, Tattersall R, eds. Adolescent and young adult rheumatology in clinical practice. Cham: Springer, 2019:35–46. [Google Scholar]
- 35. Barth S, Haas JP, Schlichtiger J. et al. Long-term health-related quality of life in German patients with juvenile idiopathic arthritis in comparison to German general population. PLoS One 2016;11:e0153267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Flatø B, Lien G, Smerdel A. et al. Prognostic factors in juvenile rheumatoid arthritis: a case-control study revealing early predictors and outcome after 14.9 years. J Rheumatol 2003;30:386–93. [PubMed] [Google Scholar]
- 37. Alves C, Luime JJ, Van Zeben D. et al. Diagnostic performance of the ACR/EULAR 2010 criteria for rheumatoid arthritis and two diagnostic algorithms in an early arthritis clinic (REACH). Ann Rheum Dis 2011;70:1645–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shaw KL, Southwood TR, McDonagh JE; British Society of Paediatric and Adolescent Rheumatology, Children’s Chronic Arthritis Association, Lady Hoare Trust for Physically Disabled Children and Arthritis Care. Growing up and moving on in rheumatology: parents as proxies of adolescents with juvenile idiopathic arthritis. Arthritis Care Res 2006;55:189–98. [DOI] [PubMed] [Google Scholar]
- 39. Arthritis and Musculoskeletal Alliance. Standards of care for people with inflammatory arthritis. 2004. http://arma.uk.net/wp-content/uploads/2017/08/inflammatory-arthritis.pdf (January 2018, date last accessed).
- 40. Foster HE, Minden K, Clemente D. et al. EULAR/PReS standards and recommendations for the transitional care of young people with juvenile-onset rheumatic diseases. Ann Rheum Dis 2017;76:639–46. [DOI] [PubMed] [Google Scholar]
- 41. Waite-Jones JM, Swallow V.. Peer-based social support for young-people with juvenile arthritis: views of young people, parents/carers and healthcare professionals within the UK. J Pediatr Nurs 2018;43:e85–91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared upon reasonable request to the corresponding author.