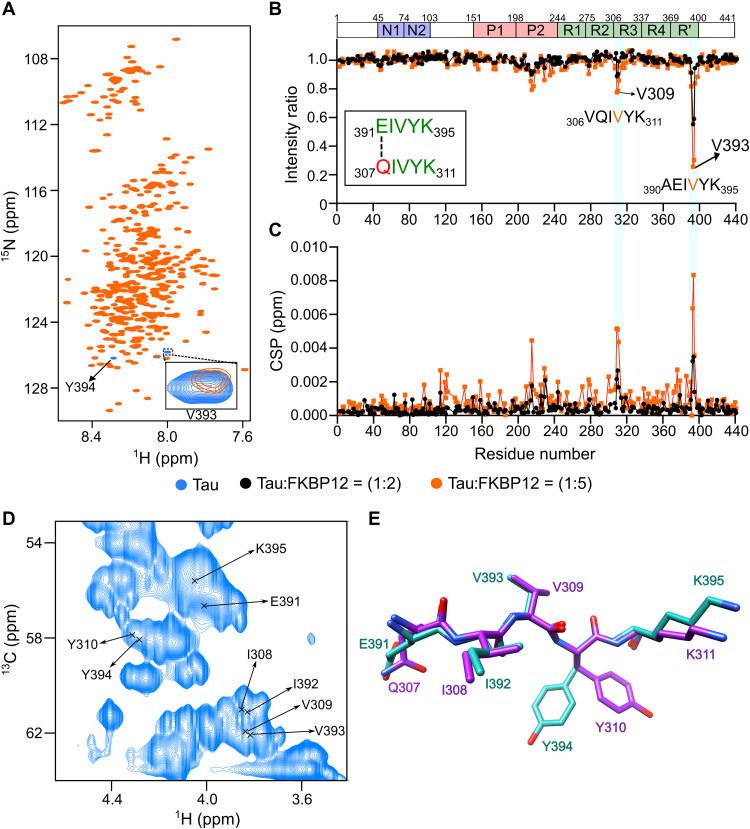
Fig. 4. FKBP12 binds a unique structural motif in monomeric tau.
(A) 2D 1H-15N HSQC spectra of tau in the absence (blue) or fivefold excess (orange) of FKBP12. The FKBP12-induced broadening of V393 is highlighted in the inset. ppm, parts per million. (B and C) Changes in the intensities (B) and chemical shift perturbations (CSPs) (C) of cross peaks in the HSQC spectra of tau upon addition of twofold (black) and fivefold (orange) excess of FKBP12. The sequence similarity of the two interaction sites of tau is displayed in the inset in (B). The domain organization of tau is shown on above. (D) Selected region of the 2D 1H-13C HSQC spectrum of tau. The Cα-Hα assignments of 391EIVYK395 and 308IVY310 are marked. Assignments of Q307 and K311 are not available. (E) Superposition of the preferred solution conformations of the two tau sequences 307QIVYK311 (purple) and 391EIVYK395 (cyan) to which FKBP12 binds.