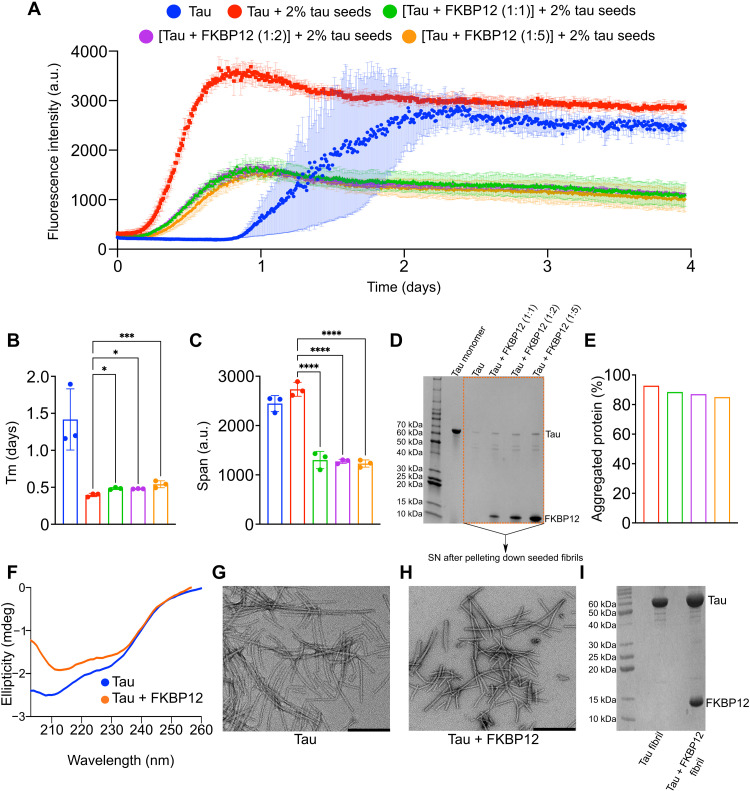
Fig. 7. FKBP12 modulates tau aggregation in vitro.
(A) Aggregation kinetics of 25 μM tau either in the absence (blue) or presence (red) of 2% preformed tau seeds. Aggregation kinetics of 1:1 (green), 1:2 (purple), and 1:5 (yellow) mixture of tau:FKBP12 in the presence of 2% preformed tau seeds. N = 3 per condition. a.u., arbitrary units. (B) Half time of aggregation (Tm) of de novo aggregated tau (blue) or seeded tau in the absence (red) or presence of equimolar (green), twofold (purple), or fivefold (yellow) molar excess of FKBP12. *P ([tau + 2% tau seeds] versus {[tau + FKBP12 (1:1)] + 2% tau seeds}) = 0.0104, *P ([tau + 2% tau seeds] versus {[tau + FKBP12 (1:2)] + 2% tau seeds}) = 0.0137, ***P = 0.0005. (C) Span of ThT intensity in the aggregation curves of de novo aggregated tau (blue) or seeded tau in the absence (red) or presence of equimolar (green), twofold (purple), or fivefold (yellow) molar excess of FKBP12. ****P < 0.0001. (D) SDS-PAGE gel of the monomeric tau protein and the supernatant (SN) of the seeded tau fibrils (after centrifugation) either in the absence or presence of equimolar, twofold, or fivefold excess of FKBP12. The fibril samples were taken after the 4 days of aggregation, as shown in (A). (E) The amount of protein aggregated. The % of aggregated proteins was determined by dividing the intensity of the SN to the monomeric tau. (F) CD spectra of tau fibrils formed without (blue) and with fivefold FKBP12 excess (orange). (G and H) Negative-stain EM image of tau fibril (G) + fivefold FKBP12 excess (H). Scale bars, 200 nm. (I) SDS-PAGE gel of tau fibrils without and with fivefold FKBP12 excess. Data are represented as means ± SD of n = 3 samples. Statistical analyses were performed by one-way ANOVA.