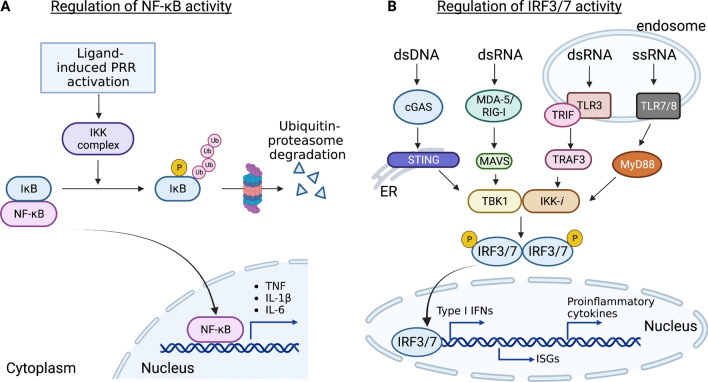
Fig. 1.
Regulation of the activation of transcription factor NF-κB and IRF3/7. A IκB is a pivotal regulator of NF-κB activity. Under homeostatic condition, NF-κB remains inactive because it is sequestered in cytoplasm by IκB. Following stimulation of PRRs, IκB proteins are rapidly phosphorylated by the IκB kinases (IKKs), resulting in the ubiquitin-mediated proteasomal degradation (Lys48) of IκB. This enables NF-κB translocation to the nucleus for transcription activation of inflammation-mediated genes such as Il6, Il1b and Tnf. B Binding of double stranded DNA (dsDNA) by cGAS results in the synthesis of cGAMP, which then binds and activates the endoplasmic reticulum resident protein STING, while sensing of viral RNA by RLRs such as RIG-1 and MDA5, activates a signaling cascade through the adapter MAVS. This event stimulates the IKK-related kinase IKK-i/TBK1, which phosphorylate IRF3/7 to induce production of type I interferons (IFNs), interferon-stimulated genes (ISGs) and proinflammatory cytokines. Similarly, TLR7/8 senses single-stranded RNA (ssRNA) in the endosome and signals through the adaptor molecule MyD88, while TLR3 recognizes double-stranded RNA (dsRNA) and uses the adaptor protein TRIF to further activate TRAF3 and IKK-i/TBK1 and the phosphorylation of IRF3/7 and the nucleus translocation to activate IFN expressions. This figure is made with BioRender: https://biorender.com/