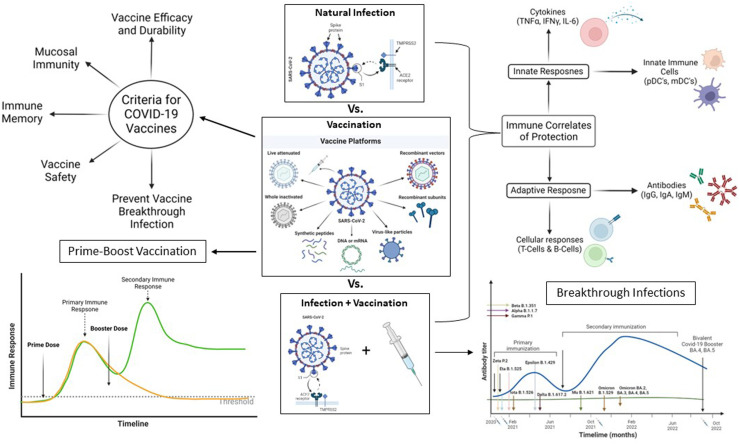
Abstract
Vaccines against SARS-CoV-2 have transformed the course of the COVID-19 pandemic with more than 30 authorizations. More than 2 billion people have been vaccinated with these vaccines developed on very different manufacturing platforms. We have reviewed the unprecedented work done in various aspects of the authorized vaccines and listed three potential improvements: 1) long-term stability at room-temperature conditions; 2) suitability for diverse populations such as infants, elderly, immune-compromised, and those with pre-existing or ongoing diseases; and 3) ability to act against different strains. In this article, we have discussed the current status of COVID-19 vaccines with respect to 1) diversity (strength and breadth) of initial immune responses and long-term immune memory; 2) prime-boost combinations that induce protection against variants; and 3) breakthrough infections. Further, we have listed host, product (critical quality attributes), and viral pathogenic factors that contribute to safety, efficacy, and effectiveness of vaccines. In addition, we have elaborated on the potential to (develop models and) determine the immune correlates that can predict long-term immune memory. The graphical representation of the abstract is provided as Fig. 1.
Keywords: SARS COV2, Immune response, Formulations, Delivery, Vaccines
Introduction
During the COVID-19 pandemic, several safe and effective vaccines against diverse SARS-CoV-2 strains were developed at an unprecedented pace. More than 30 vaccines using five major platforms have been authorized globally, and an additional 300 vaccines against SARS-CoV-2 are in development.1 , 2
The clinical symptoms of COVID-19 infection are diverse and continuously evolving as different variants of SARS-CoV-2 keep emerging, despite the advent of vaccines designed to cover these novel strains.3 The nature of immune responses, the precise signature of the protective response, and long-term safety effects of vaccines can only be fully understood after extensive long-term clinical studies of vaccinated individuals and careful studies of breakthrough infections.4 , 5 The urgent need for a COVID-19 vaccine was met with speed, successfully curbing the pandemic, but rare adverse effects are only being observed now after mass administration.6
In this report, we have summarized the nature of the immune responses induced by various COVID-19 vaccines developed using different platforms and formulations. We have elaborated on the diversity of vaccine-induced immunological memory in response to various prime-boost regimens. Further, we have commented on the level of protection or lack thereof that could result in breakthrough infections and reviewed the adverse effects of vaccines that have impacted overall safety of vaccination. For each of the sections, we have listed unmet requirements of the currently authorized vaccines that need to be fulfilled by the next generation of vaccines, (Fig. 1 ).
Figure 1.
Graphical visualization of Abstract. The current landscape of SARSCOV2 vaccines based on multiple modalities and the measures of protective immunity and the immune cells involved in propagation of immune response. The considerations for next generation of vaccines by considering the identified gaps are also presented.
Vaccine Platforms and Formulations
Various candidate vaccines have been developed using the platforms shown in Table 1 , which lists their antigen types, adjuvants, delivery platforms, dose regimen, administration routes, and storage conditions. The clinical endpoint these vaccines are required to achieve is protection against severe disease and mortality and eventually, herd immunity.7 At the time of this writing (December 2022), there are over 300 different candidate vaccines under development to prevent or ameliorate COVID-19 infections.8 Depending on geographical and economic conditions, some vaccines may not be practical due to the extreme low temperatures required for their shipping, handling and storage.
Table 1.
Summary of recently developed vaccine candidates.
| Vaccine Platform | Manufacturer | Type of Antigen | Dose of antigen | Adjuvant | Delivery Platform | Dosing Regimen | Route of administration | Storage Temperature | CITATION |
|---|---|---|---|---|---|---|---|---|---|
| mRNA-1273 (Spikevax) | Moderna | Nucleoside mRNA encoding the pre-fusion stabilized Spike glycoprotein (S) of SARS-CoV-2 virus | 100 ug | Lipid particles | 2 doses of 0.5 ml each 1 month apart | IM | -50°C to -15°C | https://www.fda.gov/media/155675/download | |
| BNT162b2 (Comirnaty) (Purple cap: To be diluted; Gray cap: Not to be diluted; some excipients differ) | Pfizer/BioNTech | Nucleoside-modified mRNA encoding the viral spike (S) glycoprotein of SARS-CoV-2 | 30 ug | Lipid particles | 2 doses of 0.3 ml each 3 weeks apart | IM | -90°C to -60°C | https://www.fda.gov/media/151707/download; https://www.fda.gov/media/154834/download | |
| JNJ-78436735 (Ad26.CoV2.S) | Janssen | Replication-incompetent recombinant adenovirus type 26 (Ad26) vector expressing the SARS-CoV-2 spike (S) protein in a stabilized conformation |
5 × 10^10 virus particles | Viral vector | one dose of 0.5 ml | IM | 2°C to 8°C | https://www.fda.gov/media/146304/download | |
| ChAdOx1-S (Vaxzevria) | Oxford (Mfd by AstraZeneca) | Chimpanzee Adenovirus encoding the SARS-CoV-2 Spike glycoprotein ChAdOx1-S | 2.5 × 10^8 infectious units | Viral vector | 2 doses of 0.5 ml each 4 to 12 weeks apart | IM | 2°C to 8°C | https://www.ema.europa.eu/en/documents/product-information/vaxzevria-previously-covid-19-vaccine-astrazeneca-epar-product-information_en.pdf | |
| Covishield | Oxford (Mfd by Serum Institute of India) | Chimpanzee Adenovirus encoding the SARS-CoV-2 Spike glycoprotein ChAdOx1-S | 5 × 10^10 virus particles | Viral vector | 2 doses of 0.5 ml each 4 to 12 weeks apart | IM | 2°C to 8°C | https://www.seruminstitute.com/pdf/covishield_ChAdOx1_nCoV19_corona_virus_vaccine_insert.pdf | |
| Covaxin | Bharat Biotech | Whole-virion inactivated SARSCoV-2 antigen (Strain: NIV-2020-770) | 6 ug | 250 ug aluminum hydroxide gel, 15 ug TLR 7/8 agonist (imidazoquinolinone) | - | 2 doses of 0.5 ml each 4 weeks apart | IM | 2°C to 8°C | https://www.bharatbiotech.com/images/covaxin/covaxin-pack-insert.pdf |
| NVX-CoV2373 (Nuvaxovid) | Novavax (Biological active subtances mfd by Serum Institute of India) | SARS-CoV-2 spike protein | 5 ug | Matrix-M: Fraction-A (42.5 ug) and Fraction-C (7.5 ug) of Quillaja saponaria Molina extract | - | 2 doses of 0.5 ml each 3 weeks apart | IM | 2°C to 8°C | https://www.ema.europa.eu/en/documents/product-information/nuvaxovid-epar-product-information_en.pdf |
| BBIBP-CorV (Covilo) | Sinopharm (Beijing) | Inactivated antigen of SARS-CoV-2 WIV04 strain | 200 WU | Aluminium hydroxide | - | 2 doses of 0.5 ml each 3-4 weeks apart | IM | 2°C to 8°C | https://www.fda.gov.ph/wp-content/uploads/2021/08/Product-Information-of-SinoPharm-Covid-19-Vaccine-Covilo.pdf |
| CoronaVac | Sinovac | Inactivated SARS-CoV-2 CZ02 strain | 600 SU | Aluminium hydroxide | - | 2 doses of 0.5 ml each 4 weeks apart | IM | 2°C to 8°C | https://www.covidvaccine.gov.hk/pdf/CoronaVac_ENG_PI_brief.pdf |
| Sputnik V (Gam-COVID-Vac) | Gamaleya National Research Center (different mfr in different countries) | Component I contains Recombinant adenovirus serotype 26 particles containing the SARS-CoV-2 protein S gene | 1.0±0.5 × 10^11 Particles | - | Viral vector | first component I at a dose of 0.5 ml, then after 3 weeks component II at a dose of 0.5 ml. | IM | -18°C or below | https://www.fda.gov.ph/wp-content/uploads/2021/03/12.-Proposed-Philippine-package-insert-Instruction-Eng.pdf |
| Component II contains Recombinant adenovirus serotype 5 particles containing the SARS-CoV-2 protein S gene | 1.0±0.5 × 10^11 Particles | ||||||||
| Sputnik Light | Gamaleya National Research Center (different mfr in different countries) | Component I contains Recombinant adenovirus serotype 26 particles containing the SARS-CoV-2 protein S gene | 1.0±0.5 × 10^11 Particles | Viral vector | Single dose of 0.5 ml | IM | -18°C or below | https://sputnikvaccine.com/about-vaccine/sputnik-light/ |
Considerations for new vaccine platforms. The precise nature of the protective immune response against SARS-CoV-2 is not completely understood. Induction of humoral and cellular immune responses is dependent on protein antigen processing and presentation by MHC class I and MHC class II molecules, which in turn drive CD8 and CD4 positive T cells. Non-protein antigens (glycans, lipids) can be presented through non-classical pathways such as CD1 and MR1, which activate cells such as Natural-killer-T (NKT) cells, γδ T cells, Mucosal Associated Immune T (MAIT) cells, and Innate Lymphoid (ILC) cells.9 The ability of a given vaccine platform to activate these cells should be investigated to better understand the mechanism by which these vaccines induce protective immune responses. Machine learning-based algorithms are being used extensively to understand diverse input parameters that lead to various clinical outcomes.10 Nevertheless, as we do not understand the exact nature of immune activation and protection, we do not know which vaccine platform is the most effective. Therefore, it may prove best to target vaccine platforms that induce a wider array of neutralizing antibodies and more robust cell-mediated immune responses, blocking infection and curbing the progression of disease. Along these lines, next-generation vaccine platforms—including pox-viral vectors, attenuated and inactivated virus, vaccine-replicating-competent vector, virus-like particles, nucleic-acid vaccines, dendritic cells and artificial antigen-presenting cells-modified with lentiviral vectors—are being evaluated for inducing protective immune responses.11 Combinations of homologous and heterologous boosters which can induce diverse immune responses are also in clinical development.6 In the December 2022 issue of NEJM, Gilbert et al have analyzed clinical efficacy of pivotal trials across the four major vaccine platforms (mRNA, protein, human adenovirus and chimpanzee adenovirus vector) to determine a universal correlate of protection. The authors have reported the potential of neutralizing antibody responses to correlate with protection, which can be used to predict severe disease.12
Diversity of Immune Responses and Long-Term Immune Memory
The diversity of immune responses to SARS-CoV-2 strains during viral infection is associated with multiple factors such as structural and non-structural viral proteins, genetic diversity of MHC Class I and II genes in the population, immune robustness, and co-morbidities of infected individuals.13 , 14 In healthy individuals, these responses culminate into responses attributed to a mix of CD8 cytotoxic T cells, CD4 effector T cells, and antibody-secreting B cells. Upon vaccination, the immune attributes that contribute to the depth and breadth of antibody responses to vaccines include: 1) generation of antibodies directed against class I, II, III, and IV regions of the receptor-binding domain (RBD) of the spike protein; 2) antibody responses to other viral antigens that are not represented in the vaccine strain; 3) somatic mutations that indicate persistent cell division and evolution of memory B cells; 4) improvement in neutralizing potency; 5) expanded clones of diverse subtypes of memory B cells; 6) memory B cell clones directed to viral antigens and variants that were not present in the vaccine; and 7) epistatic interactions between new viral mutations and pre-existing strains.15, 16, 17 A diverse array of human leucocyte antigen (HLA) associations for conserved and immunodominant SARS-CoV-2 epitopes leading to activation and expansion of diverse T cell receptor repertoire have been reported.18 , 19
Diversity of immune responses. Protection against SARS-CoV-2 is comprised of multiple immune factors. While induction of effector mechanisms such as neutralizing antibodies and CTLs has been demonstrated to be induced by the vaccines, other pathways such as antibody-dependent cellular cytotoxicity, complement-dependent cytotoxicity, induction of broadly neutralizing antibodies, activation of non-T cell responses, and the kinetics of cytokines and cytokine receptors have not yet been completely understood. This heterogeneity in protection may be related to differences in immune cell repertoire and diversity of the protection endowed by Class I and Class II HLAs within a population to the conserved non- structural domains of the virus.20 The breadth of immune responses is exemplified by the expansion of distinct CDR3 expression on T and B cell receptors; the range of T helper cell-induced cytokine responses such as Th1, Th2, Th9, and Th17; and the diversity of viral antigen-specific T and B cell responses. In addition, mucosal immune responses, the role of gut and lung microbiota, and circadian rhythms should be understood.21 Extensive analysis of these factors will enable a better understanding of protective immune responses to the vaccines and inform future vaccine development processes.22
Induction of immune memory. The induction of long-term immune memory is the hallmark of vaccines, and is established through a long, complex mechanism. First, appropriate arrays of viral antigens activate optimal signal transduction pathways in various immune cells. These pathways activate a battery of transcription factors which in turn regulate the expression of several genes, culminating in immune memory and protection from future viral antigens. Various types of immune memory, such as innate memory, central memory, and effector memory, have been described for T and B cells.23 The role of persistent antigens, long-term location in the bone marrow, and senescence in the liver have been extensively studied to describe the life cycle of memory cells. Such detailed investigative studies following vaccinations for the COVID-19 vaccine can provide better clues in understanding the mechanisms involved in the induction and persistence of long-term memory.15 , 16 Comparative analysis of different vaccine platforms and appropriate adjuvants that induce diverse types of long-term, robust memory response would be beneficial for the development of future vaccines.
Booster Doses and Considerations for Broader Variant Coverage
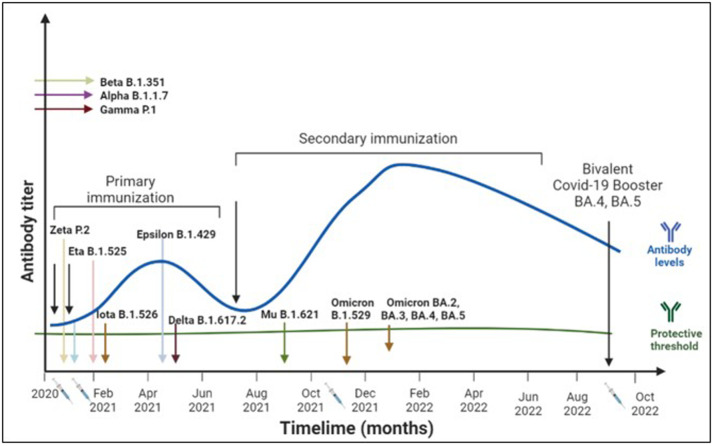
Booster doses of vaccine can ensure the longevity of immune memory responses.24 , 25 Fig. 2 shows the immune response to primary, secondary, and tertiary vaccinations. Several studies have shown that the third dose generates higher immune responses compared to the first two doses.24 Additionally, the antibodies produced by the B cells induced after the third dose recognize a wider range of variants. Furthermore, heterologous boosters induce stronger immune responses than homologous ones,26 and longer duration between the primary and booster doses has been reported to enhance and lengthen immune responses. Due to increased breakthrough infections, the next generation of booster vaccines directed against the variant strains has been authorized for emergency use.27
Figure 2.
Timeline of variants, vaccines, and protection against COVID-19. Several COVID-19 variants emerged over time around the globe. In US, the primary (two-dose regimen) resulted in protective immune response. However, with time this immune response waned off and immunization with a third dose helped boost the immune responses, however, the durability of this immune response is yet to be determined.
Prime-boost regimens of vaccinations have been established to greatly enhance immune responses, both quantitatively and qualitatively.24 The strength of immune responses can be exemplified by several types of memory T and B cells, the extent of innate and adaptive cytokine responses, and the diversity of cellular and humoral factors in mucosal immune responses. Understanding the precise nature of immune pathways involved in generation of long-lasting immune memory cells that can elicit protective immune response may inform the dosing and schedule of homologous and heterologous boosters. The mechanisms involved in enhanced immune responses are dependent on the nature of booster doses using homologous vectors (and antigens) versus heterologous vectors. The correlations between the two could be elucidated using approaches like design of experiment (DOE), and neural network of systems immunology.23 , 28 As stated above, induction of the diverse immune responses that confer long-term immune memory against different strains of the virus can be studied by investigation of different prime boost regimens.
Breakthrough Infections
When individuals vaccinated with prime and booster vaccine regimens still get infected with the virus, it is known as breakthrough infection.5 Effective vaccination prevents reinfection by inducing cell mediated immune responses which clear circulating virus and virus-infected cells and prevent reinfection. Recent studies have extensively reported on the mechanisms of breakthrough infection.4 , 5 Some of these findings include i) emergence of new variants that bypass the neutralizing ability of antibodies, ii) decreased immunity induced by the vaccine, and iii) sub-optimal immune response of the individual due to co-morbid conditions. The Centers for Disease Control (CDC) monitors reported vaccine breakthrough infections in its Morbidity and Mortality Weekly Reports.29 One of the goals of studying breakthrough infections is to determine the protective level of antibodies (and other immune correlates) that are required to confer complete protection from viral infection. Extensive studies conducted over several decades have reported these protective levels for other viruses such as Hepatitis B and Varicella.30 Understanding the lower limits of this multi-factorial immune correlate will be critical in inducing sterilizing immunity and preventing breakthrough infections in populations.31
Immune factors for booster doses. SARS-CoV-2 has been mutating extensively through the course of the pandemic.32 Each strain of the virus is the result of escape from immune pressure of the vaccines. The evolution of the virus through mutations, even though random, enables the survival of the fittest strain. Immune responses induced by a vaccine that can cover multiple strains could elicit the breadth of protection that can protect against the various mutating strains of the virus. The next generation of vaccines should anticipate future mutations of the virus and identify regions in the framework that are conserved across virus strains, so that vaccines can be developed and administered prior to the epidemic that may ensue due to the mutated strain.33 Such an approach is currently in use with influenza viruses.34 On the other hand, a universal vaccine that elicits a broad range of neutralizing antibodies and protective T cell responses can protect against all upcoming strains of the virus.35 Both new strain-specific and universal vaccines against SARS-CoV-2 can prevent further spread of the pandemic or epidemics. The threshold of immune responses that protects against infection is multi-factorial, involving both cell-mediated immunity (CD4 and CD8 T cell responses) and humoral immunity (neutralizing antibody responses). The determination of this multi-factorial threshold of protection can be established by systematically measuring immune responses in vaccinated subjects who are susceptible to SARS-COV2 infection. Such studies to determine the level of protection are conducted using meta-analysis of clinical trial data, and real-world-data mined from a larger number of subjects.36 , 37
Vaccine Safety and Considerations for Future Generation of Safe Vaccines
Adverse events from vaccination are the basis for vaccine hesitancy and vaccine suspicion. Most vaccines have an array of adverse events, several of which are identified and monitored through controlled clinical trials, a risk management plan, pharmacovigilance, and transparent safety reporting processes. Several billion doses of the COVID-19 vaccines have been administered in more than 180 countries. The major serious lethal adverse events reported include thrombosis-related myocarditis and anaphylaxis.38 Vaccine-induced immune thrombocytopenia and thrombosis (VITT) were recently reported in 266 individuals, among 31 million doses administered (∼10 million subjects), with a bias towards younger recipients.38 Several mechanisms of action for these adverse events have been reported: i) thrombosis-induced plaques, ii) cytokine storm-induced disseminated intravascular coagulopathy, and iii) induction of anti-platelet factor–4 (PF4) autoantibodies.
Safe and effective vaccines are essential for public health. While the current vaccines have shown a high benefit-risk ratio, they are also associated with observed and unpredictable adverse events. A comprehensive risk analysis of the potential severe adverse events needs to be performed and identified risk factors need to be mitigated or managed in order to improve the next-generation vaccines. The immuno-pathological mechanisms underlying these adverse events often involve multi-factorial events, which have either direct, indirect, synergistic, or sequential effects. These must be thoroughly investigated through processes described in the ICHQ9 risk assessment and mitigation guidelines.39
Conclusion
Despite several dozen authorized vaccines to SARS-CoV-2 and its variants, more than half of the global population does not have access to safe and effective doses. Emerging viral variants have resulted in breakthrough infections, which could be either due to sequence differences in spike domains of new variants that are not covered with current vaccines or waning of vaccine-induced immunity. None of the authorized vaccines induce sterilizing mucosal protective immune responses. We have listed some of the requirements that could improve global vaccine coverage: 1) long-term stability at room-temperature conditions; 2) suitability for diverse populations such as infants, elderly, immune-compromised, and those with pre-existing or ongoing diseases; and 3) ability to act against different strains.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank Vihang Ghalsasi for copy-editing the manuscript.
References
- 1.Heaton P.M. The Covid-19 vaccine-development multiverse. N Engl J Med. 2020 doi: 10.1056/NEJMe2025111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krammer F. SARS-CoV-2 vaccines in development. Nature. 2020;586(7830):516–527. doi: 10.1038/s41586-020-2798-3. [DOI] [PubMed] [Google Scholar]
- 3.Vabret N., et al. Immunology of COVID-19: current state of the science. Immunity. 2020 doi: 10.1016/j.immuni.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mizrahi B., et al. Correlation of SARS-CoV-2-breakthrough infections to time-from-vaccine. Nat Commun. 2021;12(1):6379. doi: 10.1038/s41467-021-26672-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hacisuleyman E., et al. Vaccine breakthrough infections with SARS-CoV-2 variants. N Engl J Med. 2021;384(23):2212–2218. doi: 10.1056/NEJMoa2105000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Jighefee, H.A.-O., et al., COVID-19 vaccine platforms: challenges and safety contemplations. LID - 10.3390/vaccines9101196 [doi] LID - 1196. 2021(2076-393X (Print)). [DOI] [PMC free article] [PubMed]
- 7.Sacks D., et al. Multisociety consensus quality improvement revised consensus statement for endovascular therapy of acute ischemic stroke. Int J Stroke. 2018;13(6):612–632. doi: 10.1177/1747493018778713. [DOI] [PubMed] [Google Scholar]
- 8.Mallapaty S Fau - Callaway, E., et al., How COVID vaccines shaped 2021 in eight powerful charts. (1476-4687 (Electronic)). [DOI] [PubMed]
- 9.Mayassi T., et al. A multilayered immune system through the lens of unconventional T cells. Nature. 2021;595(7868):501–510. doi: 10.1038/s41586-021-03578-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Booth A.L., Abels E., McCaffrey P. Development of a prognostic model for mortality in COVID-19 infection using machine learning. Mod Pathol. 2020:1–10. doi: 10.1038/s41379-020-00700-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simões R.S.Q., Rodríguez-Lázaro D. Classical and next-generation vaccine platforms to SARS-CoV-2: biotechnological strategies and genomic variants. Int J Environ Res Public Health. 2022;19(4) doi: 10.3390/ijerph19042392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilbert P.B., et al. A Covid-19 milestone attained — a correlate of protection for vaccines. N Engl J Med. 2022 doi: 10.1056/NEJMp2211314. [DOI] [PubMed] [Google Scholar]
- 13.Grifoni A., et al. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell, 2020;181(7):1489–1501. doi: 10.1016/j.cell.2020.05.015. e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Polvere I, et al. Humoral immune response diversity to different COVID-19 vaccines: implications for the “green pass” policy. Front Immunolol. 2022;11(13) doi: 10.3389/fimmu.2022.833085. Front Immunol. 2022 May 11;13:833085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muecksch F., et al. Increased memory B cell potency and breadth after a SARS-CoV-2 mRNA boost. Nature. 2022;607(7917):128–134. doi: 10.1038/s41586-022-04778-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sette, A., et al., Immunological memory to SARS-CoV-2 infection and COVID-19 vaccines. LID - 10.1111/imr.13089 [doi] Breakthrough COVID-19 Infections in the US: Implications for Prolonging the Pandemic. LID - 10.3390/vaccines10050755 [doi] LID - 755 Defining the risk of SARS-CoV-2 variants on immune protection COVID-19 vaccines in patients with cancer: immunogenicity, efficacy and safety. 2022(1600-065X (Electronic)).
- 17.L, W., Epistasis lowers the genetic barrier to SARS-CoV-2 neutralizing antibody escape. bioRxiv. https://www.biorxiv.org/content/10.1101/2022.08.17.504313v1. bioRxiv, 2022. [DOI] [PMC free article] [PubMed]
- 18.Tavasolian F., et al. HLA, Immune Response, and Susceptibility to COVID-19. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.601886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nelde A., et al. SARS-CoV-2-derived peptides define heterologous and COVID-19-induced T cell recognition. Nat Immunol. 2021;22(1):74–85. doi: 10.1038/s41590-020-00808-x. [DOI] [PubMed] [Google Scholar]
- 20.Douillard, V., et al., Current HLA Investigations on SARS-CoV-2 and Perspectives. (1664-8021 (Print)). [DOI] [PMC free article] [PubMed]
- 21.Lynn, D.A.-O., et al., Modulation of immune responses to vaccination by the microbiota: implications and potential mechanisms. (1474-1741 (Electronic)). [DOI] [PMC free article] [PubMed]
- 22.Lineburg, K.E., et al., CD8(+) T cells specific for an immunodominant SARS-CoV-2 nucleocapsid epitope cross-react with selective seasonal coronaviruses. (1097-4180 (Electronic)). [DOI] [PMC free article] [PubMed]
- 23.Muroyama Y., Wherry E.J. Memory T-cell heterogeneity and terminology. Cold Spring Harb Perspect Biol. 2021;13(10) doi: 10.1101/cshperspect.a037929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barouch D.H. Covid-19 vaccines - immunity, variants, boosters. N Engl J Med. 2022 doi: 10.1056/NEJMra2206573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Addo I.Y., et al. Duration of immunity following full vaccination against SARS-CoV-2: a systematic review. Arch Public Health. 2022;80(1):200. doi: 10.1186/s13690-022-00935-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Logunov D.Y., et al. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomised phase 1/2 studies from Russia. Lancet. 2020;396(10255):887–897. doi: 10.1016/S0140-6736(20)31866-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barouch D.H. Covid-19 vaccines - immunity, variants, boosters. N Engl J Med. 2022;387(11):1011–1020. doi: 10.1056/NEJMra2206573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pedroza-Pacheco I., McMichael A.J. Immune signature atlas of vaccines: learning from the good responders. Nat Immunol. 2022;23(12):1654–1656. doi: 10.1038/s41590-022-01361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.COVID-19 vaccine breakthrough infections reported to CDC - United States, January 1-April 30, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(21):792–793. doi: 10.15585/mmwr.mm7021e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tatarewicz S.M., et al. Strategic characterization of anti-drug antibody responses for the assessment of clinical relevance and impact. Bioanalysis. 2014;6(11):1509–1523. doi: 10.4155/bio.14.114. [DOI] [PubMed] [Google Scholar]
- 31.Wahl I., Wardemann H. Sterilizing immunity: understanding COVID-19. Immunity. 2022;55(12):2231–2235. doi: 10.1016/j.immuni.2022.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.World-O-Meter. Coronavirus. 2022 21 Sep 2022]; Available from: https://www.worldometers.info/coronavirus/.
- 33.Chalkias S., et al. A bivalent omicron-containing booster vaccine against Covid-19. N Engl J Med. 2022 doi: 10.1056/NEJMoa2208343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oltz E.M. Immunity to influenza: closing in on a moving target. J Immunol. 2019;202(2):325–326. doi: 10.4049/jimmunol.1890024. [DOI] [PubMed] [Google Scholar]
- 35.Wang E., Chakraborty A.K. Design of immunogens for eliciting antibody responses that may protect against SARS-CoV-2 variants. PLoS Comput Biol. 2022;18(9) doi: 10.1371/journal.pcbi.1010563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pawaskar M., et al. Relative efficacy of varicella vaccines: network meta-analysis of randomized controlled trials. Curr Med Res Opin. 2022;38(10):1772–1782. doi: 10.1080/03007995.2022.2091334. [DOI] [PubMed] [Google Scholar]
- 37.Pillsbury M., et al. Comparison of performance of varicella vaccines via infectious disease modeling. Vaccine. 2022;40(29):3954–3962. doi: 10.1016/j.vaccine.2022.05.003. [DOI] [PubMed] [Google Scholar]
- 38.Xie Y., et al. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28(3):583–590. doi: 10.1038/s41591-022-01689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jindani R., Sheth S., Paul S., Rathore A.S., Chirmule N. Platform-specific risk assessment of COVID-19 vaccines using failure mode and effects analysis. Biopharm Int. 2021:15–20. [Google Scholar]