Figure 1.
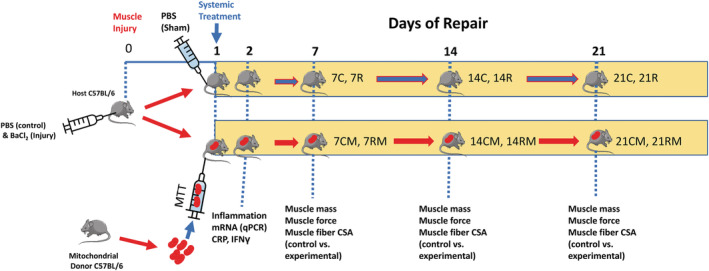
Research design. The study design is shown for the 21‐day study. Mice received phosphate‐buffered saline (PBS) injected into one gastrocnemius muscle, which acted as the non‐injured intra‐animal control. The contralateral gastrocnemius muscle was injected with BaCl2 to cause muscle injury. Animals then received a systemic injection of PBS (sham treatment) or mitochondria (mitochondrial transplant therapy, MTT) treatment through a tail vein 24 h after BaCl2 or PBS injection into the gastrocnemius muscles. In some acute experiments (2 days after muscle injury), mitochondria were stained with MitoTracker deep red prior to systemic injection, to identify if they were incorporated into the muscles of damaged mice. MTT was conducted on unstained mitochondria for experiments that were 7–21 days after muscle injury. Undamaged control muscles from PBS‐sham treated animals were harvested 7 days (7C), 14 days (14C), or 21 days (21C), after BaCl2 injury to the contralateral gastrocnemius muscle. Injured‐repairing gastrocnemius muscles from PBS‐sham treated animals were also harvested after 7 days (7R), 14 days (14R) or 21 days (21R) following the BaCl2 injury. Systemic delivery of 50 μg of mitochondria suspended in PBS was delivered via the tail vein in MTT‐treated mice. Intra‐animal control (PBS‐injected, non‐damaged) muscles of MTT‐treated mice were designated as control, mitochondria treated (CM) muscles. The CM muscles were examined after 7 days (7CM), 14 days (14CM) or 21 days (21CM), after BaCl2 or PBS injection. Repairing BaCl2‐injured muscles from MTT‐treated animals (RM) were harvested after repair of 7 days (7RM), 14 days (14RM) or 21 days (21RM) following BaCl2 injury. Outcome measures included muscle force, fatigue, muscle weight, fibre cross‐sectional area and the percentage of collagen and other non‐contractile tissue in injured‐repaired and control non‐injured muscles. Forty‐eight hours after injury a subset of mice were examined for muscle inflammatory markers and plasma was collected for assessment of IFN‐γ and C‐reactive protein as markers for systemic inflammation.
