Figure 7.
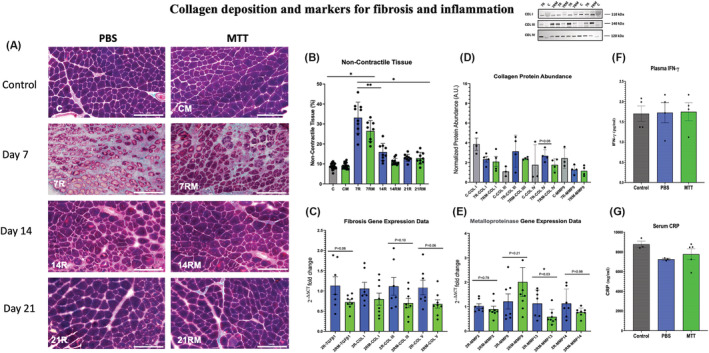
Collagen deposition and markers for fibrosis and for inflammation. (A) Gomori's trichrome non‐contractile collagen staining. Examples of tissue cross sections of control and injured gastrocnemius muscles after phosphate‐buffered saline (PBS) or mitochondrial transplant therapy (MTT) treatment. Collagen fibres were identified with Gomori's trichrome staining in gastrocnemius tissue cross sections from PBS‐treated or MTT‐treated mice after 7 days (7R,7RM), 14 days (14R, 14RM) or 21 days (21R, 21RM) of injury. The white bar is 200 μm in length. (B) Quantification of the non‐contractile and collagen tissue. Collagen deposition was quantified stereologically by a 224‐point count grid and the percentage of the total fibre tissue section was presented as mean ± SD. Control non‐injured PBS sham‐treated muscles (C), and MTT‐treated muscles (CM). PBS repairing muscles 7 (7R), 14 (14R) and 21 days (21R) after injury and mitochondria‐treated repairing muscles after 7 (7RM), 14 (14RM), 21 (21RM) days after injury. *P < 0.05, versus 7R and 7 RM; **P < 0.05, Day 7R versus Day 7RM. (C) Collagen/fibrosis gene markers. Gastrocnemius muscles were injured by BaCl2 and injected with PBS or MTT 24 h after the muscle injury. mRNA was isolated from muscles 48 h after the initial injury. The relative expression levels of amplified transcripts were estimated by the comparative CT method (2−ΔΔCT) and normalized to a house keeping gene (GAPDH). The temperature cycle profile for the qPCR reactions was 50°C for 2 min 95°C for 10 s followed by 40 cycles of 95°C for 15 s and 60°C for 60 s. A melt curve of 95°C for 15 s, 60°C for 60 s, and 95°C for 15 s was used to verify the specificity of the amplified PCR product. The data were expressed as fold‐differences compared with the uninjured intra‐animal control muscle. mRNA expression was measured by qPCR for genes that are indicators of fibrosis and collagen signalling (TGFβ1). The temperature cycle profile for the qPCR reactions was 50°C for 2 min 95°C for 10 s followed by 40 cycles of 95°C for 15 s and 60°C for 60 s. A melt curve of 95°C for 15 s, 60°C for 60 s, and 95°C for 15 s was used to verify the specificity of the amplified PCR product. The data were expressed as fold‐differences compared with the uninjured intra‐animal control muscle. mRNA expression was measured by qPCR for genes that are indicators of fibrosis and collagen signalling (COL I, COL III and COL V). The data are expressed as the fold change of 2−ΔΔCT from injured as compared with control muscles. (D) Collagen protein abundance. The gastrocnemius muscle of one limb was injured by BaCl2, and the contralateral limb was injected with PBS which did not cause muscle injury. Twenty‐four hours after the injury, mice received a systemic injection of PBS or MTT. Approximately 40 mg of muscle was homogenized, and cell lysates were separated on SDS‐polyacrylamide gels then transferred to polyvinylidene fluoride (PVDF) membranes. The membranes were blocked in 5% bovine serum albumin (BSA) and total protein was fluorescently quantified via the iBright FL1500 imaging system (Invitrogen). The membranes were then incubated overnight at 4°C with primary antibodies for Collagen I (alpha 2(I) chain with a predicted molecular weight ~110 kDa), Collagen III (molecular weight ~140 kDa) or Collagen IV (molecular weight ~120 kDa). The membranes were washed and incubated with anti‐rabbit or anti‐mouse IgG‐conjugated secondary antibodies then the signals were developed with SuperSignal West Pico plus ECL reagent and imaged. The proteins were then quantified by ImageJ. The data show that MTT had a tendency (P = 0.08) to suppress Collagen IV protein abundance in injured‐repairing muscles, 7 days after injury. An example of western blots for Collagen I (COL I), Collagen III (COL 3) and Collagen IV (COL IV) are shown in the insert. (C) Control uninjured muscles; 7R, PBS‐treated muscles, 7 days after injury; 7RM, MTT‐treated muscles, 7 days after injury. (E) Metalloproteinase mRNA. Transcript levels were determined for metal metalloproteinases (MMP2, MMP9, MMP13 and MMP14) to determine if MTT affected collagen/fibrosis remodelling during muscle repair after an injury. The gastrocnemius muscle of one limb was injured by BaCl2 injection, while the opposite limb received PBS as a non‐injury control. Twenty‐four hours after injury, mice received a systemic injection of PBS or MTT. The muscles were harvested, and mRNA was isolated from muscles 48 h after the initial injury. The relative expression levels of amplified transcripts were estimated by the comparative CT method (2−ΔΔCT) and normalized to a house keeping gene (GAPDH). The temperature cycle profile for the qPCR reactions was 50°C for 2 min 95°C for 10 s followed by 40 cycles of 95°C for 15 s and 60°C for 60 s. a melt curve of 95°C for 15 s, 60°C for 60 s, and 95°C for 15 s was used to verify the specificity of the amplified PCR product. The data were expressed as fold differences compared with the uninjured intra‐animal control muscle. This suggests that signalling for fibrosis/non‐contractile tissue tended to have lower mRNA in injured muscles from MTT‐ than PBS‐treated animals 2 days of injury. *P < 0.05, versus 2R MMP13 versus 2RM MMP13. All other data failed to reach significance. The P values shown on the figure. (F) Plasma levels of IFN‐γ. Plasma levels of IFN‐γ were measured by ELISA in control naive non‐injured mice (n = 4), and 48 h after systemic injection of either PBS (n = 4) or MTT (n = 4). Data are presented as mean ± SD. Mice did not receive a muscle injury in these experiments, to identify whether MTT created a systemic inflammatory response in the mice. No increases in IFN‐γ were identified by systemic delivery of MTT. (G) C‐reactive protein (CRP). Plasma levels of CRP were measured as a second marker of systemic inflammation. CRP was detected by an ELISA in control naive non‐injured, non‐treated mice (n = 3), and 48 h after systemic injection into the tail vein mice for PBS (n = 3) or MTT (n = 4). No increase in CRP was identified after systemic delivery of mitochondria to the mice 2 days after systemic injection, as compared with PBS‐treated or non‐treated animals.
