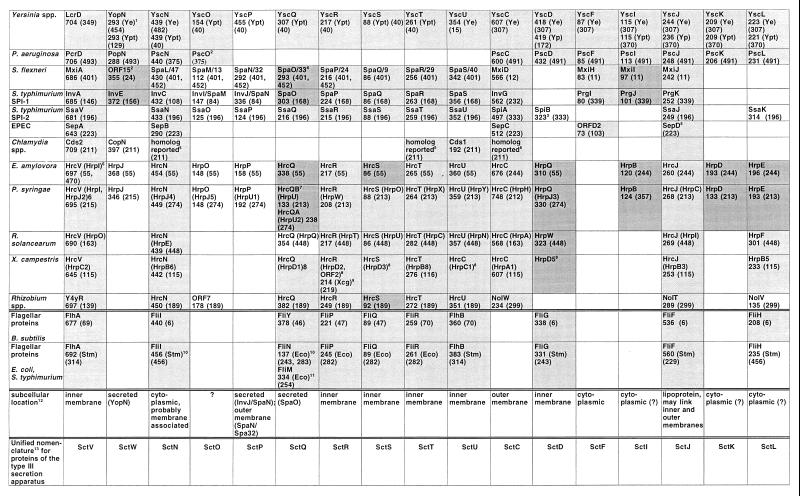
FIG. 10.
Broadly conserved proteins of the type III secretion apparatus and of the flagellum biosynthesis systems in B. subtilis and E. coli/S. typhimurium. The lengths of the proteins in amino acids given underneath the protein designation. Numbers in parentheses indicate references. Subgroups of proteins which exhibit significant similarity are indicated by different grades of shading. See the text for further details. 1, Abbreviations in parentheses denote the organism. Ye, Y. enterocolitica; Ypt, Y. pseudotuberculosis; Yp, Y. pestis. 2, Sequence from Shigella sonnei. In S. flexneri, only part of the respective MxiC sequence is available. 3, Sequence incomplete. 4, In SpaO and other members of the YscQ family, only the carboxy-terminal 80 aa is conserved, while SpaO also shares an internal domain (aa 147 to 194) with SpaO from S. typhimurium. 5, Sequence not available. 6, Older designations are given in parentheses. 7, HrcQB corresponds to the conserved carboxy-terminal domain of other YscQ-family members, while the HrcQA corresponds to the amino-terminal domain of the conserved HrcQ from E. amylovora (Fig. 11). 8, U. Bonas, unpublished. 9, Xcg, X. campestris pv. glycinea. 10, Stm, S. typhimurium; Eco, E. coli. 11, FliM of E. coli is homologous to the amino terminus of B. subtilis FliY. See the text and Fig. 11 for further details. 12, In most cases, the subcellular location is predicted according to the physicochemical properties of the protein or inferred from the location of homologous flagellar biosynthesis proteins. 13, Unified nomenclature for conserved proteins of the type III secretion apparatus. Sct stands for secretion and cellular translocation.