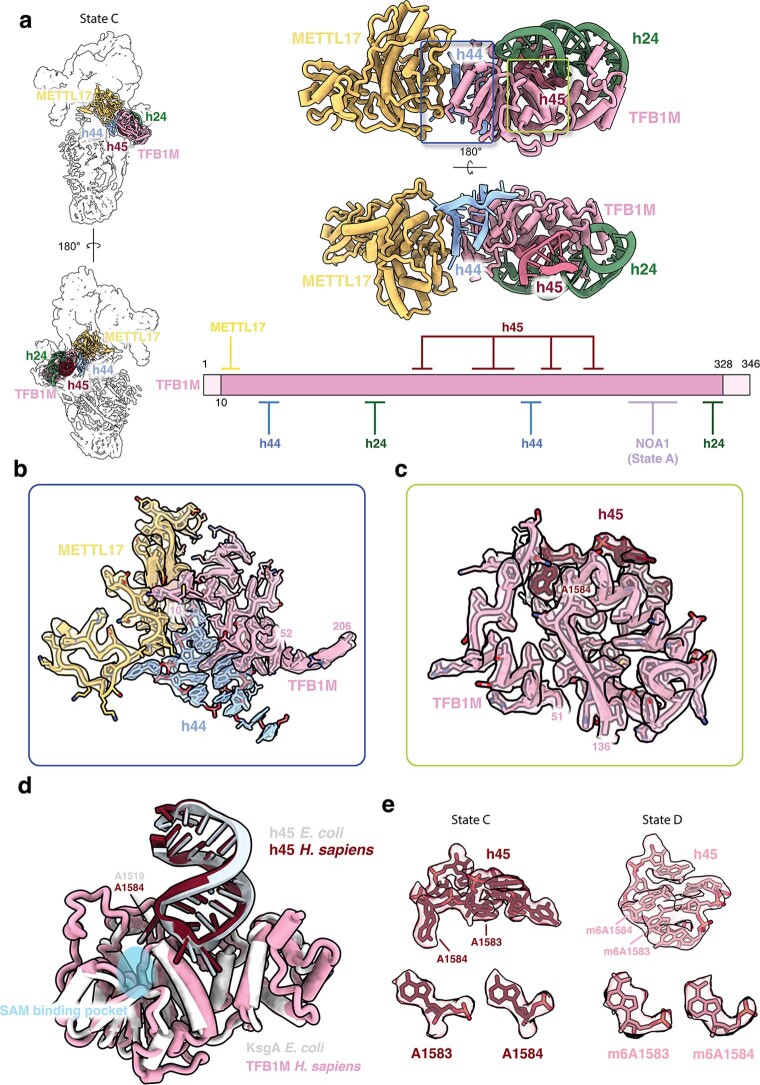
Extended Data Fig. 3. TFB1M plays roles in h44 maturation and h45 methylation.
(a) Interaction network of TFB1M in State C in context with the entire complex (left), shown in cartoon (right) and in a schematic (bottom). (b) Models and cryo-EM maps showing TFB1M h44 clamping activity. (c) Models and cryo-EM maps showing the enzymatic active site lacking density for methyl donor S-adenosyl methionine. (d) Comparison of h45 binding mode between TFB1M and its bacterial homologue KsgA (PDB:7O5H)39. (e) Comparison of h45 cryo-EM density between State C and State D, showing conformational changes and appearance of density corresponding to modified adenosine residues. Maps from both states are displayed at the same contour level.