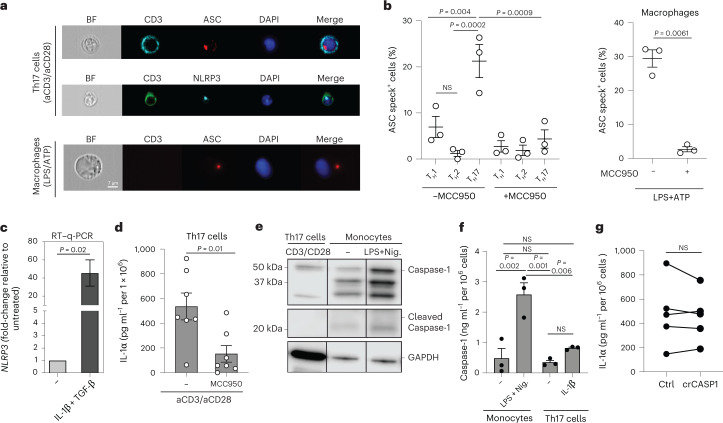
Fig. 5. Unconventional NLRP3 inflammasome activation regulates IL-1α production by human TH17 cells.
a,b, Imaging flow cytometry with TH17 cells on day 5 after stimulation with plate-bound anti-CD3 and anti-CD28 monoclonal antibodies and macrophages after 24 h of stimulation with LPS and ATP for the last 30 min. a, Representative experiment. BF, bright-field. b, Cumulative data with n = 3 biological samples, presented as mean ± s.e.m. Left, P values calculated using one-way ANOVA with Tukey’s multiple-comparison test. Right, P values calculated using two-tailed, paired Student’s t-test. c, RT–qPCR analysis of TH17 cells stimulated as in a and restimulated with PMA and ionomycin for 3 h. Data represent three independent experiments with n = 9 biological replicates (two-tailed, paired Student’s t-test). d, ELISA of cell culture supernatants after stimulation of TH17 cells for 5 d with anti-CD3 and anti-CD28 monoclonal antibodies. Data represent three experiments with n = 7 biological replicates (two-tailed, paired Student’s t-test). e, Immunoblot analysis of cell lysates from TH17 cells after 5 d of stimulation with anti-CD3 and anti-CD28 monoclonal antibodies and of monocyte lysates after stimulation with LPS for 24 h and nigericin (Nig.) for the last 30 min. The conditions from the same blot after removal of irrelevant conditions or replicates are shown. f, ELISA of cell culture supernatants from anti-CD3- and anti-CD28-activated TH17 cells (5 d) and LPS (24 h)- and nigericin (30 min)-stimulated monocytes (n = 3 biological samples presented as mean ± s.e.m.; one-way ANOVA with Tukey’s multiple-comparison test). g, ELISA of cell culture supernatants from anti-CD3- and anti-CD28-activated TH17 cells after depletion of CASP1 by CRISPR–Cas9 gene editing. Data represent five independent experiments. P values were calculated using two-tailed, paired Student’s t-test. Each circle indicates an independent blood donor.