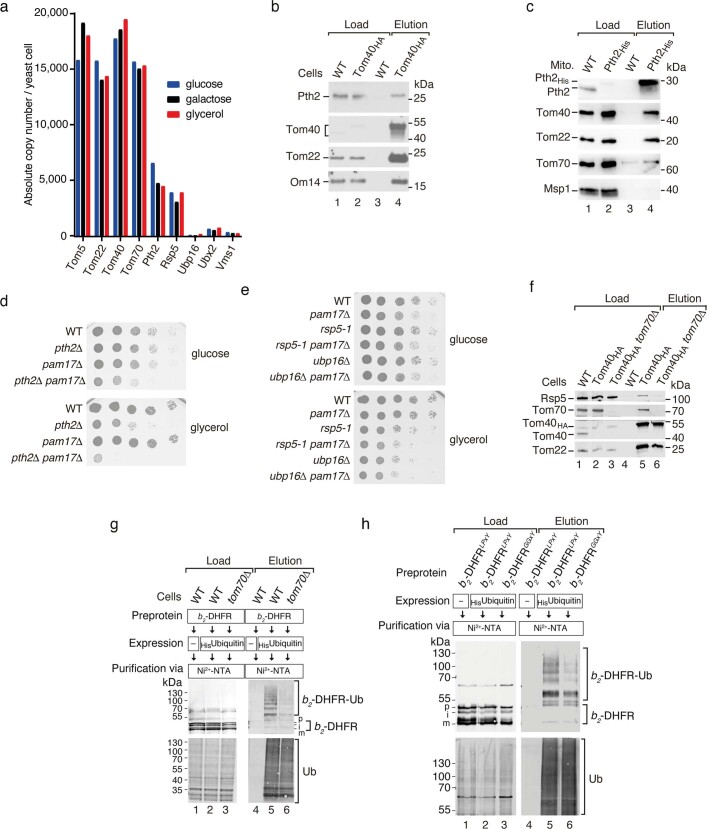
Extended Data Fig. 9. Characterization of Pth2, Ubp16 and Rsp5.
a, Absolute copy number per yeast cell of the indicated proteins. Values are based on the quantification of mitochondrial proteins grown on different carbon sources by Morgenstern et al.6. b, Wild-type (WT) and Tom40HA cell extracts were lysed with digitonin and subjected to affinity purification. Proteins were analysed by SDS-PAGE and immunodetection with the indicated antisera. Load 1%, elution 100%. c, WT and Pth2His mitochondria were lysed with digitonin and subjected to affinity purification via Ni-NTA agarose. Proteins were analysed by SDS-PAGE and immunodetection with the indicated antisera. Load 0.2% (Tom40, Tom22, Tom70) or 2% (Pth2, Msp1), elution 100%. Control protein: Msp1, mitochondrial sorting of proteins 1. The precursor of Pth2 is imported from the cytosol via the receptor Tom70 and inserted into the outer membrane by the mitochondrial import complex MIM21. d, Serial dilutions of the indicated strains were spotted onto full medium with either glucose or glycerol as carbon source and grown at 30 °C. e, Serial dilutions of the indicated strains were spotted onto full medium containing glucose or glycerol as carbon source and grown at 24 °C. f, Rsp5 preferentially binds to substrates and adaptor proteins via interaction with a PPxY/LPxY motif31,91–93. Tom70 contains a PPxY motif (aa 71–74) and may thus be involved in recruiting Rsp5 to mitochondria. WT, Tom40HA and Tom40HA tom70∆ cell extracts were lysed with digitonin and subjected to affinity purification. Proteins were analysed by SDS-PAGE and immunodetection with the indicated antisera. Load 0.2%, elution 100%. The interaction of Rsp5 with the TOM complex is impaired in the absence of Tom70. g, WT and tom70∆ cells expressing b2-DHFR and His-tagged ubiquitin as indicated were lysed under denaturing conditions and subjected to affinity purification via Ni-NTA agarose. Proteins were analysed by SDS-PAGE and immunodetection with antisera against the DHFR domain (upper panel) or ubiquitin (lower panel). b2-DHFR-Ub, ubiquitin modified b2-DHFR. Load 0.2%, elution 100%. Precursor ubiquitylation is diminished in the absence of Tom70. h, We found an LPxY motif in the DHFR domain of b2-DHFR. WT yeast cells expressing the standard precursor cytochrome b2-DHFRLPxY or the mutant form b2-DHFRGGxY and His-tagged ubiquitin as indicated were lysed under denaturing conditions and subjected to affinity purification via Ni-NTA. Proteins were analysed by SDS-PAGE and immunodetection with the indicated antisera. b2-DHFR-Ub, ubiquitin modified b2-DHFR. Input 0.2%, elution 100%. Ubiquitylation of the mutant precursor was reduced. These data suggest that Tom70 is involved in recruiting Rsp5 to the mitochondrial import site. Rsp5 may use Tom70 as an adapter for interacting with precursor proteins and, in case a precursor substrate contains a PPxY/LPxY motif, also engage the motif on the precursor.