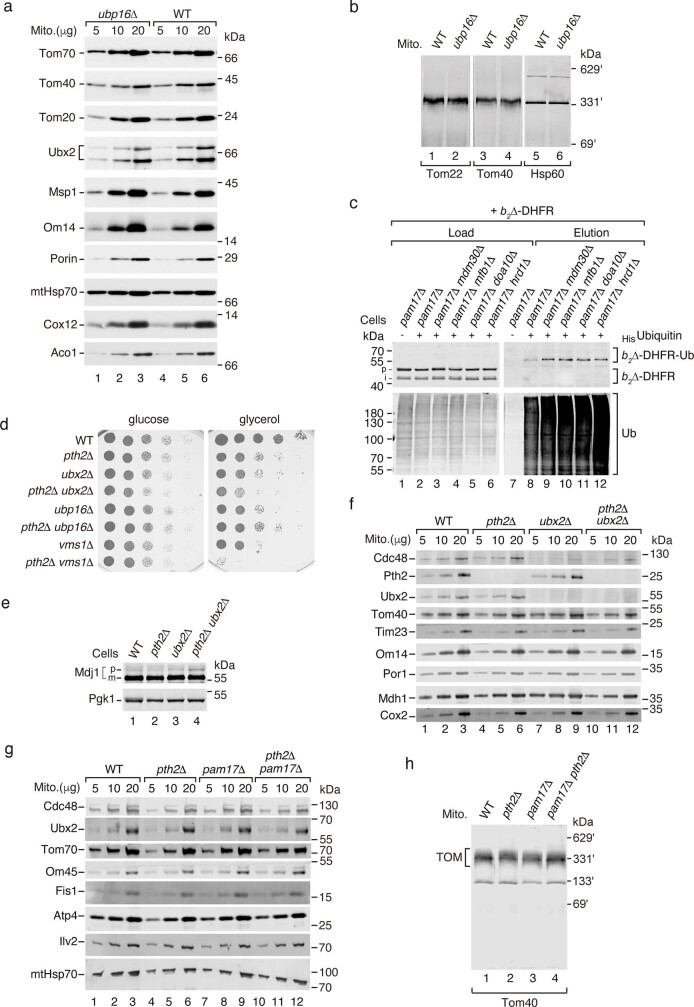
Extended Data Fig. 10. Characterization of yeast cells and mitochondria lacking Ubp16, Pth2 or Ubx2.
a, The indicated protein amounts of WT and ubp16∆ mitochondria were analysed by SDS-PAGE and immunodetection with the indicated antisera. b, WT and ubp16∆ mitochondria were lysed with digitonin and analysed by blue native electrophoresis and immunodetection using an antiserum against Tom22, Tom40 and Hsp60. The levels of TOM, Ubx2 and further tested proteins (a) were not altered in ubp16∆ mitochondria, excluding that the lack of Ubp16 led to indirect effects on the protein levels of import or mitoTAD components. c, WT and the indicated strains expressing cytochrome b2∆-DHFR and His-tagged ubiquitin as indicated were lysed under denaturing conditions and subjected to affinity purification via Ni-NTA. Proteins were analysed by SDS-PAGE and immunodetection with the indicated antisera. b2∆-DHFR-Ub, ubiquitin modified b2∆-DHFR. Input 0.2%, elution 100%. d, Serial dilutions of the indicated strains were spotted onto full medium with either glucose or glycerol as carbon source and grown at 30 °C. e, Cell extracts of wild-type (WT), pth2∆, ubx2∆ and pth2∆ ubx2∆ strains were analysed by immunodetection with the indicated antisera. p, precursor; m, mature form of Mdj1. The single deletions of Pth2 and Ubx2 did not lead to an accumulation of the Mdj1 precursor when the complete mitochondrial import machinery including Pam17 was present (see also Fig. 3d)17. However, the Mdj1 precursor moderately accumulated in pth2∆ ubx2∆ double mutants, supporting the view that a fraction of preproteins accumulate even when the mitochondrial import system is fully active94–98. Thus, at least one of the pathways, Ubx2 or Pth2, has to be active for removal of accumulated precursor proteins. f, The indicated protein amounts of WT, pth2∆, ubx2∆ and pth2∆ ubx2∆ mitochondria were analysed by SDS-PAGE and immunodetection with the indicated antisera. g, The indicated protein amounts of WT, pth2∆, pam17∆ and pth2∆ pam17∆ mitochondria were analysed by SDS-PAGE and immunodetection with the indicated antisera. h, WT, pth2∆, pam17∆ and pth2∆ pam17∆ mitochondria were lysed with digitonin and analysed by blue native electrophoresis and immunodetection with Tom40 specific antisera. The steady state levels of Ubx2, Cdc48 and TOM are not substantially altered in pth2∆ and pth2∆ pam17∆ mutant mitochondria, excluding that the accumulation and ubiquitylation of precursor proteins (Fig. 3d, e) is indirectly caused by a loss of mitoTAD or TOM components. Yeast strains, plasmids and antisera are listed in Supplementary Tables 4–6 (refs. 99–102).