Figure 5. Possible modes of lipid interactions with transmembrane proteins.
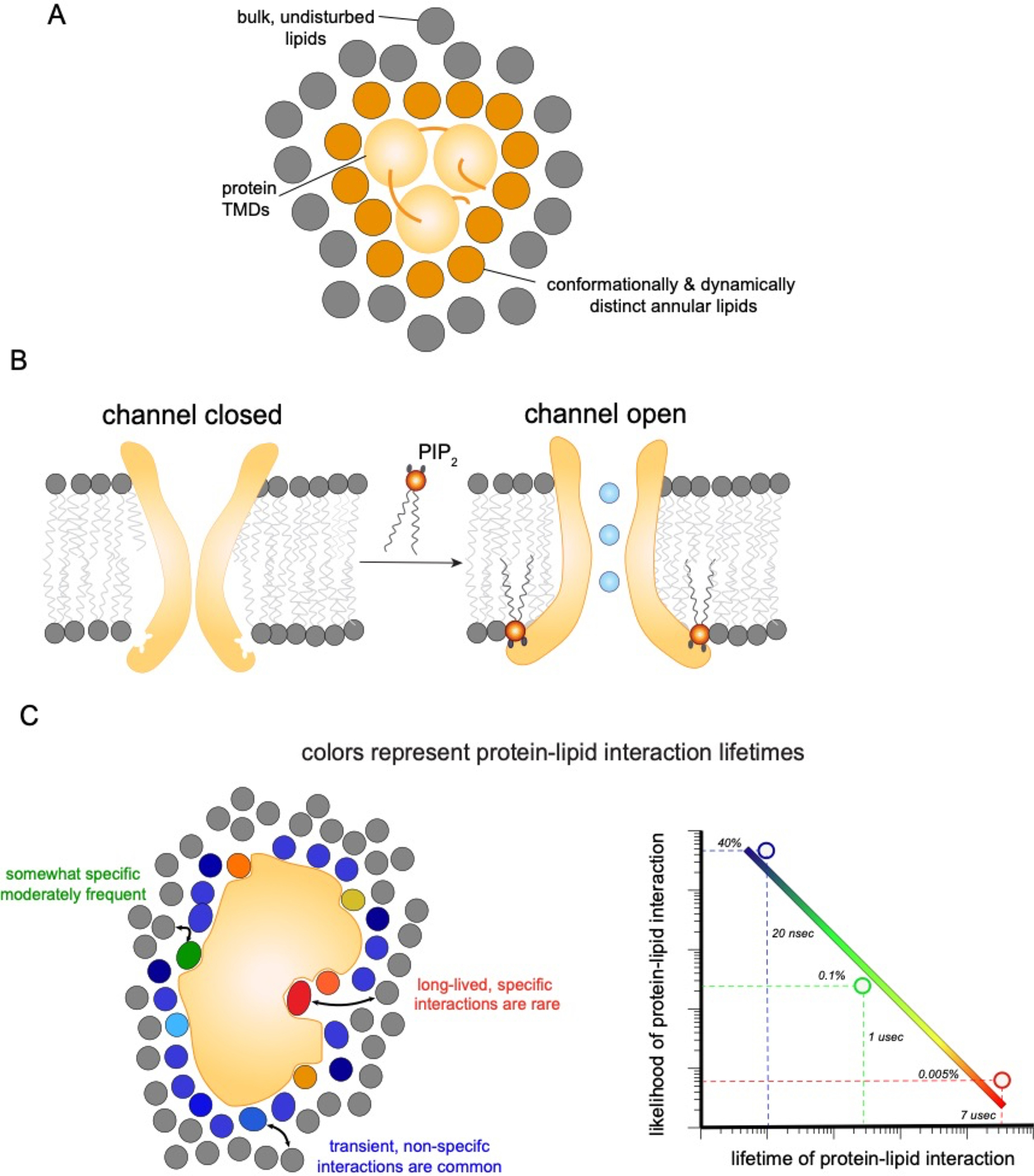
(a) A shell of annular lipids stably associates with transmembrane protein regions. The exchange rate of these annular lipids with the bulk is slow, such that these lipids can be conceptualized as an extended part of the protein. (b) Charged and large lipids like PIP2 (orange), can be tightly and specifically bound by protein domains to introduce large scale conformational changes. In these cases, lipids act as allosteric ligands for protein function. (c) Interactions between IMPs and individual lipid molecules (colored) are in constant competition with those from bulk lipids (gray). The affinity for a particular lipid species determines how likely it is to occupy a particular site, relative to its bulk concentration. The lifetime of those interactions is directly related to affinity. Simulations suggest that protein-lipid interactions span a range of lifetimes from relatively high affinity interactions (which are rare) to very common short-lived interactions (nanosecond range), which indicate rapid lipid exchange not influenced by protein binding. These observations imply that there is no single characteristic scale for protein-lipid interactions, but rather that specific, semi-selective, and bulk solvent-like effects could potentially be simultaneously relevant.
