Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection which is commonly known as COVID-19 (COronaVIrus Disease 2019) has creeped into the human population taking tolls of life and causing tremendous economic crisis. It is indeed crucial to gain knowledge about their characteristics and interactions with human host cells. It has been shown that the majority of our genome consists of non-coding RNAs. Non-coding RNAs including micro RNAs (miRNAs) and long non-coding RNAs (lncRNAs) display significant roles in regulating gene expression in almost all cancers and viral diseases. It is intriguing that miRNAs and lncRNAs remarkably regulate the function and expression of major immune components of SARS-CoV-2. MiRNAs act via RNA interference mechanism in which they bind to the complementary sequences of the viral RNA strand, inducing the formation of silencing complex that eventually degrades or inhibits the viral RNA and viral protein expression. LncRNAs have been extensively shown to regulate gene expression in cytokine storm and thus emerges as a critical target for COVID-19 treatment. These lncRNAs also act as competing endogenous RNAs (ceRNAs) by sponging miRNAs and thus affecting the expression of downstream targets during SARS-CoV-2 infection. In this review, we extensively discuss the role of miRNAs and lncRNAs, describe their mechanism of action and their different interacting human targets cells during SARS-CoV-2 infection. Finally, we discuss possible ways how an interference with their molecular function could be exploited for new therapies against SARS-CoV-2.
Keywords: COVID-19, LncRNA, MiRNA, ncRNA, SARS-CoV-2
1. Covid-19
The Coronaviridae family comprises of seven (HCoV-229E, HCoV-NL63, HCoV-OC43, HCoV-HKU1, MERS-CoV, SARS-CoV, SARS-CoV-2) familiar human coronaviruses (CoV). Among them, four (HCoV-229E, HCoV-NL63, HCoV-OC43, HCoV-HKU1) cause mild to moderate respiratory infections while three (MERS-CoV, SARS-CoV, SARS-CoV-2) are known to cause severe illness and fatality (Devarakonda et al., 2021). Genome sequencing revealed that SARS‐CoV‐2 shows high similarity (70–80%) to SARS‐CoV and 40% to the Middle East respiratory syndrome MERS‐CoV (Lu et al., 2020b, Perlman, 2020). SARS-CoV led to an outbreak of severe acute respiratory syndrome (SARS) in China near late 2002 while MERS-CoV was responsible for the cause of Middle East respiratory syndrome (MERS) in 2012. As seen, coronavirus outbreaks are not new on the horizon, but the COVID-19 pandemic caused by SARS-Cov-2 virus which initially began in Wuhan, China in late 2019 and spread extensively worldwide (Lai et al., 2020) has shown an unprecedented increase in the infection and mortality rates, as compared to previous coronaviruses outbreaks.
1.1. Structure and pathophysiology of SARS-CoV-2
The name coronavirus is derived from a Latin word ‘corona’ meaning crown. In fact, coronaviruses exhibit a crown like morphology due to the presence of surface spike (S) protein when investigated under the electron microscope. The S protein is unique and of relative importance as it has several proteases to cleave it (Belouzard et al., 2012). The S proteins act as a mediator for the virus to enter the host cell via binding to Angiotensin-converting enzyme 2 (ACE2) receptor (Li et al., 2003). The S protein is composed of glycoproteins and comprises of S1 and S2 subunits. The S2 subunit is highly conserved along with fusion peptide, transmembrane domain, and cytoplasmic domain (Beeraka et al., 2021). Coronaviruses are enveloped and single-stranded positive-sense RNA with the length of the genetic material of the SARS-CoV-2 being 30 kb, making it the largest RNA virus ( Fig. 1 ) (Hussain et al., 2005). The coronavirus genomes including SARS-CoV-2 encode five major open reading frames (ORFs). ORF1a and ORF1b are located at 5′ end of the genome and are among the largest as they contain majority of the genetic material. ORF1a and ORF1b possess protease activity and are responsible for cleaving the polyproteins into a variety of non-structural proteins (nsp) which furthermore facilitates viral transcription, replication and eventually modulates host transcription and translation (Nomburg et al., 2020). The remaining ORF encodes four canonical 3′ structural proteins including the spike (S), envelope (E), membrane (M) and nucleocapsid (N) proteins and are commonly found in all coronaviruses (Ashour et al., 2020, Michel et al., 2020).
Fig. 1.
SARS-CoV-2 protein and genomic structures. Schematic representation of SARS-CoV-2 structure. SARS-CoV-2 virus is an enveloped and single-stranded RNA with the length of the genetic material of 30 kb. The genome is composed non-structural and structural proteins. The non-structural protein is encoded from two Open Reading frames: ORF1a and ORF1b located at the 5′ end. Other ORFs located at the 3′ end encodes structural proteins including the Spike (S), Envelope (E), Membrane (M), Hemagglutinin esterase-dimer, and the Nucleocapsid (N).
The coronavirus begins its life cycle initially by attaching to the host cell’s ACE2 receptor through the S protein. Subsequently, they get into the host cells via endocytosis or membrane fusion (penetration) where they release viral contents enabling viral RNA to enter the nucleus for further replication (Yuki et al., 2020). Finally, biosynthesis of viral proteins using viral mRNA take place followed by their maturation and release of viral proteins (Yuki et al., 2020, Abu-Izneid et al., 2021) ( Fig. 2 ).
Fig. 2.
Coronavirus life cycle. After invading body tissues, SARS-CoV-2 starts its life cycle by attaching ACE2 host receptor. The life cycle of the virus with the host consists of the following steps: 1) attachment, 2) penetration (endocytosis), 3) RNA release, 4) biosynthesis, 5) maturation (Virus Assembly) and 6) virion release.
2. Micro RNA (miRNA): an introduction
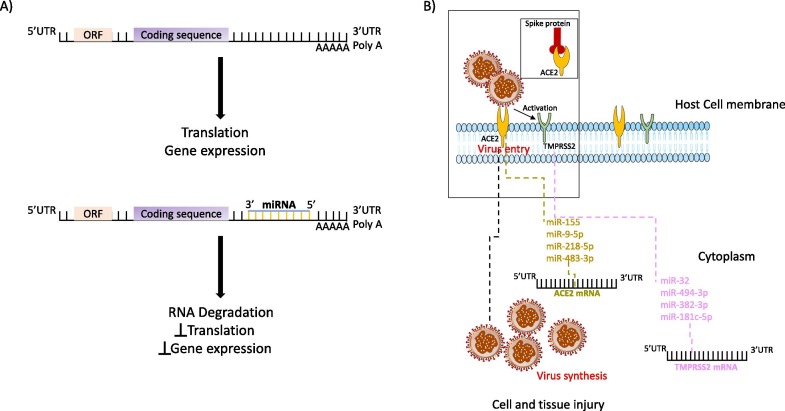
Among the non-coding class of RNAs, miRNAs are well conserved and exist in most eukaryotes. Although miRNAs are small in length (approximately 18–25 nucleotides), they play a significant role in the control and regulation of gene expression. The well-known function of miRNAs is the silencing of RNA and the regulation of post-transcriptional cellular gene expression that ultimately control several biological processes like differentiation, proliferation, apoptosis etc. (Gebert and MacRae, 2019, Pottoo et al., 2020). Human miRNAs downregulate the target genes generally through binding to their 3′UTR resulting in the suppression or degradation of mRNAs (Bartel, 2009, Gutbrod and Martienssen, 2020) ( Fig. 3 A). As miRNA utilizes their short seed sequence (2–7 nucleotides) at the 5′ end to form a complementary pairing with the mRNAs, a single miRNA can regulate several different genes using the cell's RNA interference machinery (Gutbrod and Martienssen, 2020). These miRNAs have been estimated to regulate almost 60% of human protein coding genes and serve as diagnostic biomarkers or as therapeutic targets (Wang and Szeto, 2007, Friedman et al., 2009, Kato et al., 2013, Ramezani et al., 2015, Bhatt et al., 2016, Rossato et al., 2017, Fu et al., 2020). Given the broad spectrum of miRNA functions, it is not surprising that miRNAs are involved in the regulation of viral infections and host defense (Sanders et al., 2011, Nguyen et al., 2018, Zhao et al., 2018a, Bandopadhyay and Bharadwaj, 2020, Li et al., 2020b).
Fig. 3.
Interaction between miRNAs and SARS-CoV-2. A) miRNA binds to the 5′UTR, 3′UTR of genome and the coding sequences of proteins within the mRNA by base pairing leading to RNA degradation, inhibiting translation and gene expression. B) miRNA targeting SARS-CoV-2 host entry receptors ACE2 (Angiotensin-converting enzyme 2) and TMPRSS2 (serine protease serine 2) expression.
2.1. A quick glance at miRNAs for host-virus interaction
miRNAs are considered as crucial players in host-virus interactions. There are several evidence highlighting the involvement of miRNAs in viral pathogenesis process (Trobaugh and Klimstra, 2017, Girardi et al., 2018, Zhao et al., 2018b, Zheng et al., 2018). miRNAs alter host immune system against the virus, either by promoting viral replication or altering miRNA mediated host gene regulation (Trobaugh and Klimstra, 2017). The host miRNAs have been found to interact with the viral RNA by binding to the 5′UTR, 3′UTR of viral genome and the coding regions (CDS) of viral proteins (Zheng et al., 2013, Trobaugh et al., 2014, Girardi et al., 2018). The consequences of binding to the 3′UTR resulted in either the suppression or increased RNA viral translation (Abu-Izneid et al., 2020); to the 5′UTR promoted viral replication by the stabilization of virus RNA (Girardi et al., 2018, Abu-Izneid et al., 2020); and binding to the coding region of viral-mRNA led to the inhibition of viral replication by suppressing the translation of viral genes (Girardi et al., 2018, Abu-Izneid et al., 2020). Recent findings have shown the role of miRNA against hepatitis B virus and the collaboration of miRNA 494-3p to assist Epstein-Barr virus in host immune escape (Zhao et al., 2018a, Bandopadhyay and Bharadwaj, 2020, Li et al., 2020b). Ingle et al. utilized microarray-based miRNA profiling in human cells infected with Newcastle disease virus (NDV) and found that miR-485-5p was upregulated both in cells with NDV infection as well as in cells infected with Influenza A virus (IAV) H5N1 (Ingle et al., 2015). Huang and group (Song et al., 2010) showed that cellular miRNAs like miR-491, miR-654 and miR-323 inhibit replication of the H1N1 Influenza A virus. Smith et al. also identified several antiviral miRNAs like miR-34, miR-15 and miR-517 against flaviviruses (Dengue virus, West Nile virus, Zika virus) (Smith et al., 2017). Lodge et al. identified miR-221 and miR-222 as negative regulators of CD4 expression and CD4-mediated HIV-1 entry, suggesting that MiR-221/miR-222 function as effectors of the host antiviral response that are triggered during macrophage infection and block HIV-1 entry (Lodge et al., 2017). Triboulet et al. reported the suppression of polycistronic miRNA cluster miR-17/92 by HIV-1 for proper viral replication which was dependent on the histone acetyltransferase Tat cofactor PCAF (Triboulet et al., 2007). miR-122 is a well-studied miRNA associated with hepatitis C and is specifically expressed and most abundant in human liver (Jopling et al., 2005). Host miR-122 leads to increased viral replication by direct miRNA-virus interaction with the hepatotropic virus (a positive-sense ssRNA virus) (Jopling et al., 2005, Trobaugh and Klimstra, 2017). A detailed study by Landford et al. demonstrated the use of miR-122 in the treatment of chronic hepatitis C in primates (chimpanzees) (Lanford et al., 2010). Several clinical trials have shown the efficacy of anti miR-122 (drug named as Miravirsen) against hepatitis C (Janssen et al., 2013, van der Ree et al., 2014). Further improvement for the drug delivery in hepatocytes with significant reduction in viral load was achieved by N-acetylgalactosamine conjugated antisense oligonucleotide for miR-122, known as RG-101 (van der Ree et al., 2014). However, anti-miR-122 approach using RG-101 has been discontinued due to the identification of high levels of bilirubin in the blood, which was confirmed from the pre-clinical and clinical investigation (van der Ree et al., 2017). Hsu et al. also revealed miR-122 as a tumor suppressor in liver that questions the use of antagomir against miR-122 (Hsu et al., 2012).
2.2. MiRNAs and coronaviruses
Several studies have shown the involvement of miRNAs in coronaviruses infections ( Table 1 ). Using differential miRNA expression analyses by next generation sequencing (NGS), plenty of miRNAs were found to be differentially expressed by coronaviruses like Transmissible gastroenteritis virus (TGEV), Severe acute respiratory syndrome (SARS), Middle east respiratory syndrome coronavirus (MERS), Porcine epidemic diarrhea virus (PEDV) (Mallick et al., 2009, Peng et al., 2011, Ma et al., 2018a, Lin et al., 2019, Kemp et al., 2020). Ma et al. found 65 microRNAs to be differentially expressed in Mock- and TGEV-infected intestinal porcine epithelial cell-jejunum 2 (IPEC-J2) cell line (Ma et al., 2018a). In another study, miR-4331 acts as an oncogene inducing mitochondrial damage during TGEV infection by suppressing Retinoblastoma 1 (RB1), promoting interleukin-1 receptor accessory protein (IL1RAP), and activating p38 MAPK pathway (Zhao et al., 2018b). Liu and his group showed that during TGEV infection, there is decrease in the level of miR-30a-5p which is a negative regulator of Interferons (IFN) signaling pathway: cytokine signaling protein 1 (SOCS1) and SOCS3 (Ma et al., 2018b). miR-200c-3p has been shown to play a critical role in acute respiratory distress syndrome (ARDS) by downregulating angiotensin converting enzyme ACE2 during infection by SARS and pneumonias (Liu et al., 2017). Peng et al. utilized an integrative deep sequencing of severe acute respiratory syndrome coronavirus (SARS-CoV) infected mice lung samples and revealed differential expression of 45 mature miRNAs during SARS-CoV or influenza virus infection (Peng et al., 2011). Human coronavirus OC43 is known to be a major contributor to the common cold worldwide (McIntosh et al., 1967, Birch et al., 2005, Lai et al., 2014). However, little research has been done on OC43 due to its low mortality rate (Patrick et al., 2006). One of the studies focusing on OC43 revealed the effects of its nucleocapsid on the transcription factor NF-κB (Lai et al., 2014). Mechanistically it was shown that OC43 nucleocapsid binds to miR-9, a negative regulator of NF-κB, resulting in prolonged activation of NF-κB responsible for immune evasion for this virus (Lai et al., 2014). This study (Lai et al., 2014) could be explored for the mechanisms (focusing on nucleocapsid protein and miR-9) by which other coronaviruses like SARS-CoV-2 possibly evade the human immune system.
Table 1.
miRNAs associated with SARS-CoV-2 infections.
| miRNA | Regulation | Expression | Reference |
|---|---|---|---|
| miR-223 | Regulated by nucleocapsid | Downregulated | (Mallick et al., 2009) |
|
miR-98 miR-335-5p miR-26b-5p |
Regulated by spike protein |
Downregulated |
(Mallick et al., 2009) (Teodori et al., 2020) (Teodori et al., 2020) |
|
miR-141/miR-200 let-7e/miR-125a |
ACE2 |
Downregulated |
(Nersisyan et al., 2020) |
|
miR-200c-3p miR-200c miR-18 miR-9-5p miR-218-5p miR-483-3p |
Regulation of ACE2 |
Upregulated |
(Bozgeyik, 2021) (Liu et al., 2018, Fu et al., 2019, Kozak et al., 2020, Saberinia et al., 2020) (Zhang et al., 2018) (Pierce et al., 2020) (Pierce et al., 2020) (Pierce et al., 2020) |
| miR-140-3p | Regulated by TMPRSS2 | Downregulated | (Kim et al., 2020) |
|
miR-214 miR-98 miR-32 let-7d-5p miR-494-3p miR-382-3p miR-181c-5p |
Targeting TMPRS2 mRNA |
Upregulated |
(Kaur et al., 2021) (Kaur et al., 2021) (Kaur et al., 2021) (Pierce et al., 2020) (Pierce et al., 2020) (Pierce et al., 2020) (Pierce et al., 2020) |
|
miR-17-5p miR-20b-5p miR-323a-5p |
Antiviral effect |
– |
(Khan et al., 2020) |
|
miR-519-3p miR-18b-5p miR-1304-5p miR-367-3p |
Viral miRNA |
– |
(Guterres et al., 2020) |
|
miR-93 miR-17 |
Regulates IL-8 |
Upregulated |
(Fabbri et al., 2014) (Oglesby et al., 2015) |
|
miR-146a-5p miR-21-5p miR-142-3p miR-181a-2-3p miR-31-5p miR-99a-5p miR-183-5p miR-627-5p miR-144-3p |
Circulating miRNA |
Downregulated |
(Tang et al., 2020a) (Tang et al., 2020a) (Tang et al., 2020a) (Tang et al., 2020a) (Tang et al., 2020a) (Tang et al., 2020a) (Li et al., 2020a) (Li et al., 2020a) (Li et al., 2020a) |
|
miR-15b-5p miR-486-3p miR-486-5p miR-16–2-3p miR-6501-5p miR-618 |
Circulating miRNA |
Upregulated |
(Tang et al., 2020a) (Tang et al., 2020a) (Tang et al., 2020a) (Li et al., 2020a) (Li et al., 2020a) (Li et al., 2020a) |
| miR-530b-5p | Plant derived; targeted the ribosomal slippage site between ORF1a and ORF1b of SARS-CoV-2 genome | – | (Kalarikkal and Sundaram, 2021) |
| miR2911 | Plant derived; binding to the mRNA and inhibits protein translation and virus replication | – | (Zhou et al., 2015, Li et al., 2018b, Huang et al., 2019) |
| miR-24 | Antiviral miRNAs via targeting p38 MAPK signaling pathways | – | (McCaskill et al., 2017) |
| miR-124 | Antiviral miRNAs via targeting p38 MAPK signaling pathways | – | (McCaskill et al., 2017) |
| miR-744 | Antiviral miRNAs via targeting p38 MAPK signaling pathways | – | (McCaskill et al., 2017) |
| miR-30e-5p | Increases innate immune responses | – | (Mishra et al., 2020) |
| miR-136 | Bind in the 3′ UTR of IL-6 | – | (Hou et al., 2009) |
| miR-146a | Inhibits the production of the RIG-I-dependent antiviral response by targeting TRAF6 | Upregulated | (Hou et al., 2009) |
| miR-2392 | Circulating miRNA (suppresses the mitochondrial activity) | Upregulated | (McDonald et al., 2021) |
2.3. Interaction between miRNAs and SARS-CoV-2 (COVID-19)
SARS-CoV-2 like other coronaviruses have been shown to regulate the host miRNAs that could control the viral proliferation and their wide out spread (Kemp et al., 2020) ( Fig. 3 B). There are possibilities that SARS-CoV-2 could also utilize virus-encoded miRNAs to infect the host for their own interest (Ghosh et al., 2009, Haasnoot and Berkhout, 2011, Peng et al., 2011, Neeb et al., 2022, Xu et al., 2022).
2.3.1. MiRNAs targeting SARS-CoV-2 entry receptor ACE2
Angiotensin-converting enzyme 2 (ACE2) is a transmembrane protein that facilitates the entry of virus into the host cell (Ziegler et al., 2020, Calderon-Dominguez et al., 2022). ACE2 has also been reported as an entry receptor for the spike protein of SARS-CoV-2 playing vital roles in COVID-19 infection (Li et al., 2003, Hoffmann et al., 2020, Calderon-Dominguez et al., 2022). Structure analysis using molecular modeling revealed that SARS-CoV‐2 has a higher affinity for ACE2 with flexible glycyl residues relative to SARS‐CoV which possess a rigid prolyl residue, possibly enabling SARS-CoV-2 more infectious (Chen et al., 2020b). A recent study with single-cell RNA-seq data analysis on the receptor ACE2 expression revealed significant risk to different human organs which are vulnerable to SARS-CoV-2 infection (Zou et al., 2020). Several miRNAs have been reported to regulate ACE2 expression with only few being well studied (Liu and Wang, 2019, Chen and Wang, 2020, Elemam et al., 2022). Mallick et al. demonstrated the miRNA landscape using human bronchoalveolar stem cells (BASCs) during SARS-CoV infection. In this work, they found that the viral nucleocapsid and spike protein downregulate miR-223 and miR-98, respectively within BASCs that serves to control the differentiation regulation of BASCs, activation of inflammatory chemokines, and suppression of ACE2. The work by Mallick et al. reveals the exploitation of cellular miRNA machinery by SARS‐CoV for their own advantage (Mallick et al., 2009).
Several studies using bioinformatic analyses identified miRNAs targeting ACE2. Nersisyan et al. used integrative analysis of miRNA and mRNA sequencing data and revealed that lysine-specific demethylase 5B (JARID1B), encoded by the KDM5B gene, indirectly affects ACE2 expression by downregulating the transcription of miRNA families like hsa-mir-141/hsa-miR-200 and hsa-let-7e/hsa-mir-125a (Nersisyan et al., 2020). In another study using bioinformatic tools, members of miR-200 family, particularly miR-200c-3p is predicted to be a good target for the regulation of ACE2 in respiratory system cells (Bozgeyik, 2021). The miR-200 family which consists of miR-200b, miR-200c and miR-429 is well explored miRNA cluster in several studies (reviewed in (Liu et al., 2018, Fu et al., 2019, Kozak et al., 2020, Saberinia et al., 2020)). Lu et al. validated miR-200c as a target of ACE2 (Lu et al., 2020a). Furthermore, Lu et al. showed that ACE2 was inhibited by miR-200c both at mRNA and protein levels in rat primary cardiomyocytes and in human iPSC-derived cardiomyocytes (Lu et al., 2020a). The inhibition of ACE2 by miR-200c could be exploited in patients with pre-existing cardiovascular diseases (CVDs) as SARS-CoV-2 strike them hard with severe symptoms of COVID-19 (Lu et al., 2020a). However, there are reports of contradicting effects of ACE2 in CVDs and COVID-19 infection, therefore the suppression of ACE2 by miR-200c should be carefully implemented (Guo et al., 2020). It is found that patients with SARS-CoV-2 have severe kidney adverse effects and ACE2 plays a very important role in kidney diseases (Durvasula and Shankland, 2008, Reich et al., 2008, Widiasta et al., 2020). Several ACE2 related miRNAs play vital roles in COVID-19 associated nephropathy (reviewed in (Widiasta et al., 2020)). It is shown that miR-18 was upregulated in nephropathy and regulated the expression of ACE2 (Zhang et al., 2018). The inhibition of miR-18 downregulated ACE2 expression by suppressing the Nox2/ROS pathway, indicating the importance of miR-18 that may be targeted to develop ACE2 related diseases therapies (Zhang et al., 2018), mostly in COVID-19 patients. Another recent study proposed the use of histone deacetylase (HDAC) inhibitors for COVID-19 therapy (Teodori et al., 2020). For the study (Teodori et al., 2020), the authors used bioinformatic analysis and predicted that miR-335-5p and miR-26b-5p were being regulated by viral S-protein and host ACE2 together with HDAC pathway (Teodori et al., 2020). A recent study showed that the expression of miR-32-5p and miR-1246 were altered in human COVID-19 patients as compared to asymptomatic individuals (Calderon-Dominguez et al., 2022).
Studies have shown that miR-146a and miR-155 are highly expressed in oral fluids of type 2 diabetes and chronic periodontitis patients (Radovic et al., 2018, Al-Rawi et al., 2020, Han et al., 2020). Roganović JR (Roganovic, 2020) used bioinformatic analysis to demonstrate that miR-146a and miR-155 upregulates ACE2 expression and modifies host antiviral response in diabetes and chronic periodontitis patients, which could render them more susceptible to SARS-CoV-2 infection (Roganovic, 2020). miR-155 seems to be a very promising therapeutic target for COVID-19. miR-155 has already been shown to be implicated with various virus infections and is a known regulator of immune cells (Thai et al., 2007, Zhou et al., 2010, Zeng et al., 2015, Mehta and Baltimore, 2016, Dickey et al., 2017, Badry et al., 2020). Recent research employing single-cell RNA sequencing of SARS-CoV-2 in infected human epithelial cell line Calu-3 showed significant induction of miR-155, further demonstrating that this miRNA possesses great potential to be used against SARS-CoV-2 (Emanuel et al., 2021). Pierce et al. used a combinational approach of bioinformatic prediction algorithms and miRNA profiling in order to predict host miRNAs implicated in the regulation of SARS-CoV-2 infection. Pierce et al. found three miRNAs (miR-9-5p, miR-218-5p, and miR-483-3p) targeting ACE2 mRNA. The differential expression of these three miRNAs was also shown to favor either resistance or susceptibility to SARS-CoV-2 infection. The authors also looked for RNA-seq data from Calu-3 cells infected with SARS-CoV-2 and found miR-483-3p as one of differentially expressed miRNA. These miRNAs targeting ACE2 represent potential therapeutic targets in COVID-19 patients (Pierce et al., 2020).
2.3.2. MiRNAs targeting SARS-CoV-2 entry receptor TMPRSS2
Besides ACE2, the human serine protease serine 2 (TMPRSS2) is known to be involved in the priming of spike (S) proteins to facilitate the viral entry into the host for causing SARS-CoV-2 infection (Ziegler et al., 2020) (Lodhi et al., 2021). TMPRSS2 is considered to be the main host protease that facilitates S protein activation on primary target cells and initial viral entry (Glowacka et al., 2011, Shulla et al., 2011, Hoffmann et al., 2020, Walls et al., 2020, Pierce et al., 2020, Kaur et al., 2021). Kim and his group performed expression analysis of miRNAs in SARS-CoV-2 infected hamster lung tissues (Kim et al., 2020). They identified bioinformatically five different miRNAs (hsa-miR-15a-5p, hsa-miR-15b-5p, hsa-miR-195-5p, hsa-miR-16-5p and hsa-miR-196a-1-3p) that commonly bind to Middle East respiratory-related coronavirus (MERS-CoV), SARS-CoV and SARS-CoV-2 (Kim et al., 2020). Moreover, they also looked for miRNAs that specifically bound to TMPRSS2 and examined their expression in hamster lung samples infected by SARS-CoV-2 (Kim et al., 2020). Among miR-140-3p and hsa-miR-1255b that were found to be targets of TMPRSS2, miR-140-3p showed lower expression in hamster lung samples infected with SARS-CoV-2 as compared to normal samples indicating that miR-140-3p may have some important function in SARS-CoV-2 infection (Kim et al., 2020). A recent study used bioinformatic tools and identified miR-214, miR-98 and miR-32 as specific targets of TMPRSS2 in SARS-CoV-2 (Kaur et al., 2021). The group also carried in vitro experiments in which they transfected Caco-2 cells with mimics of miR-214, miR-98 and miR-32 to overexpress their expression (Kaur et al., 2021). The overexpression of miR-214, miR-98 and miR-32 lead to the significant reduction of TMPRSS2 at protein level with miR-32 showing maximum inhibitory effect on TMPRSS2 (Kaur et al., 2021). Work by Kaur and group indicate that miR-32 has a high potential to suppress TMPRSS2 in SARS-CoV-2 promising it to be a nice therapeutic candidate against SARS-CoV-2 (Kaur et al., 2021). However further in vivo experiments are needed to validate their findings.
Pierce et al. found seven miRNAs (let-7d-5p, miR-494-3p, miR-382-3p, let-7e-5p, miR-181c-5p, miR-452-5p, and miR-1226-3p) targeting TMPRS2 mRNA that favored either resistance or susceptibility to SARS-CoV-2 infection (Pierce et al., 2020). Moreover, miR-382-3p and miR-494-3p also showed higher expression in SARS-CoV-2-resistant primary human lung fibroblasts cells (Pierce et al., 2020). RNA-seq data also revealed that miR-181c-5p and let-7d-5p targeting TMPRS2 mRNA were upregulated in Calu-3 cells infected with SARS-CoV-2 (Pierce et al., 2020). In brief, the findings by Pierce et al. displayed regulation of TMPRS2 in SARS-CoV-2 by host miRNAs and indicates that these miRNAs could plausibly be used as potential therapeutic targets or biomarkers in SARS-CoV-2 (Pierce et al., 2020). Several studies have shown that SARS-CoV-2 can also target endothelial cells (reviewed in (Evans et al., 2020, Libby and Luscher, 2020, Sardu et al., 2020)) that has been further confirmed by inspecting different organs, including lung, kidney, heart and gut during SARS-CoV-2 infection (Ackermann et al., 2020, Fox et al., 2020, Stahl et al., 2020, Varga et al., 2020). Matarese et al. bioinformatically identified miR-98-5p as a specific target of TMPRSS2 in human endothelial cells (Matarese et al., 2020). Furthermore, they also showed that miR-98-5p regulated TMPRSS2 transcriptionally in two different human endothelial cells (HMVEC-L and HUVEC) (Matarese et al., 2020). Although the study by Matarese et al. (Matarese et al., 2020) does not give an absolute picture for the effect of miR-98-5p in SARS-CoV-2, it increases a possible option for targeting TMPRSS2 via miR-98-5p during SARS-CoV-2 infection. Moreover, targeting miR-98 looks promising as it has also been shown by Kaur et al. (Kaur et al., 2021) as TMPRSS2 specific miRNAs that regulates SARS-CoV-2 entry checkpoint (Kaur et al., 2021). Neuroinvasion is well known in SARS-CoV and is believed that neurological impairment linked with SARS-CoV-2 might be due to neuroinvasion (Mukhopadhyay and Mussa, 2020). Virus including SARS-CoV-2 make an entry through the hypothalamic circuits via the olfactory bulb causing neurological diseases (reviewed in (Aghagoli et al., 2020, Lahiri et al., 2020, Pennisi et al., 2020, Wu et al., 2020)) and hypothalamic miRNAs regulating the expression of ACE2 and TMPRSS2 play an important role facilitating the entry of these virus (Lambert et al., 2014, Mukhopadhyay and Mussa, 2020). Mukhopadhyay et al. used an in silico analysis method to identify novel hypothalamic miRNAs regulating TMPRSS2 expression in SARS-CoV-2 (Mukhopadhyay and Mussa, 2020). Their approach revealed 29 hypothalamic miRNAs in which majority belonged to let-7 family of miRNAs (refer to (Mukhopadhyay and Mussa, 2020) for list of miRNAs) binding prominently to TMPRSS2 (Mukhopadhyay and Mussa, 2020). A recent study mechanistically validated miR-98-5p as a regulator of TMPRSS2 in human endothelial cells (Matarese et al., 2020). These miRNAs could be exploited for the identification of novel regulatory networks and as therapeutic targets against SARS-CoV-2 infection.
2.3.3. Interplay between the SARS-CoV-2 viruses' and host's miRNAs
The exact miRNA-mediated interplay between SARS-CoV-2 and its host is yet not clear and needs to be elucidated. In order to highlight the interplay mechanism between the SARS-CoV-2 viruses' and host's miRNAs, Khan et al. made use of computational approaches in order to reveal host and viral miRNAs along with their implications in associated functional pathways (Khan et al., 2020). They identified putative host antiviral miRNAs targeting the SARS viruses and also highlighted SARS viruses’ miRNAs targeting host genes (Khan et al., 2020). They also revealed that very few of the total targeting miRNAs have functional importance. For instance, Khan et al. identified 106 host anti-SARS-CoV-2 miRNAs but only three host miRNAs (miR-17-5p, miR-20b-5p, miR-323a-5p) displayed an antiviral effect during SARS-CoV-2 infection (Khan et al., 2020). Alisan et al. also found that miRNA-1307-3p, miRNA-1468-5p, miRNA-3611, miRNA-3691-3p, miRNA-3934-3p, miRNA-5197, and miRNA-8066 bind strongly to the SARS-CoV-2 genome and are associated with host responses and viral pathogenicity(Arisan et al., 2020). Similarly, in another study, miRNA-18b-5p, miRNA-197-5p, miRNA-338-3p, miRNA-1273d, miRNA-3154, miRNA-3935-5p, miRNA-4436a, miRNA-4661-3p, miRNA-4761-5p, and miRNA-5096 were also identified as strong regulators of ORF1a, ORF1b, ORF7a, and 'S' regions of the SARS CoV-2 genome (Hosseini and McLellan, 2020).
In one of the studies, Guterres et al. analyzed sixty SARS-CoV-2 genomes in order to identify regions with virus-encoded miRNA seed sponges that eventually makes a seed pairing to human miRNA for prevention of interaction with their native targets (Guterres et al., 2020). This prevention of interaction finally alleviates native miRNA suppression (Guterres et al., 2020). They found 34 and 45 miRNAs for positive and negative sense viral RNA respectively, with strong binding capacity to important SARS-CoV-2 genes. Few of these miRNAs include miR-519-3p, miR-18b-5p, miR-1304-5p, miR-367-3p. These miRNAs were associated with key roles in pulmonary and cardiac diseases like lung cancer, pneumonia, asthma, cardiac fibrosis (Guterres et al., 2020). Targeting these miRNAs will possibly perturb the immune response leading to the outburst of inflammatory cytokines that might change the host response to viral infection (Guterres et al., 2020). Overall, the studies by Khan et al. (Khan et al., 2020) and Guterres et al. (Guterres et al., 2020) indicate that miRNAs of both host and SARS-CoV-2 play a pivotal role in the pathogenesis and could be useful in RNA mediated therapeutics for COVID-19.
Furthermore, Karimi et al. identified thirty-nine mature sequence elements predicted to encode for miRNAs within the SARS-CoV-2 genome and the immune system, the respiratory system, and vitamin D were the three signaling pathways that were highlighted by enrichment analysis as being important in the pathogenesis of SARS-CoV-2 (Karimi et al., 2021). Pawlica et al identified a small RNA molecule designated CoV2-miR-O7a (for SARS-CoV-2 miRNA-like ORF7a-derived small RNA) that is linked to the cellular RNA interference machinery transcripts (Pawlica et al., 2021).
2.3.4. Potential role of miRNAs in COVID-19 cytokine storm
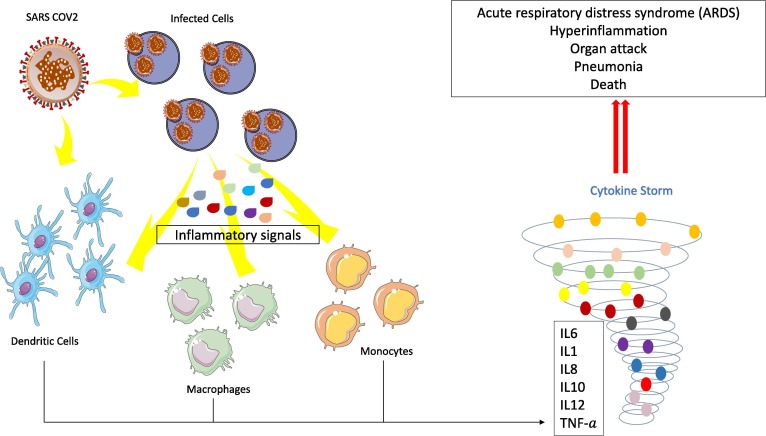
COVID-19 infection is also characterized by excessive and uncontrolled inflammatory response which leads to abrupt release of inflammatory cytokines. This particular phenomenon is termed as the cytokine storm leading to lung failure and death (Desjarlais et al., 2020) ( Fig. 4 ). Activation of pro-inflammatory genes like IL-1ß, IL-6, NF-kB, IL-8, STAT-3, TNF-α, G-CSF, MCP-1 actually triggers the cytokine storm in COVID-19 patients (Gao et al., 2020, Mehta et al., 2020, Tang et al., 2020b, Vabret et al., 2020). Several works have demonstrated that miRNAs are important regulators of gene expression of these pro-inflammatory genes (Fabbri et al., 2014, Oglesby et al., 2015, Hong et al., 2016) and could be used against the COVID-19 cytokine storm as well. Fabbri et al. showed that miR-93 post-transcriptionally regulates IL-8 in bronchial epithelial cells during Pseudomonas aeruginosa infection (Fabbri et al., 2014). In addition, they experimentally demonstrated that overexpression of miR-93 reduced IL-8 mRNA level as well as IL-8 production (Fabbri et al., 2014). Another study by Oglesby and his group reported that the overexpression of miR-17 in cystic fibrosis bronchial epithelial cells decreased IL-8 release (Oglesby et al., 2015). As mentioned before, an individual miRNA can simultaneously regulate hundreds of different targets. Therefore, it is possible that one miRNA could modulate several inflammatory signaling pathways at the same time (reviewed in (Sonkoly and Pivarcsi, 2009)). In a cohort of human chronic obstructive pulmonary disease (COPD) patient study, Hu et al. (Hu et al., 2017) showed that miR-125b positively regulated the expression of TNF-α, IL-1β, and IL-8, which are directly implicated in the acute exacerbation of COPD (Hu et al., 2017) and are mainly responsible for the cytokine storm in COVID-19. There are plenty of other studies showing the role of miRNAs in combating cytokine storm (reviewed in (Mi et al., 2013, Saba et al., 2014, Desjarlais et al., 2020, Rarani et al., 2022)). Considering the above cited examples, we can predict that miRNAs are going to be a useful therapeutic tool and as a covering shield against the cytokine storm of COVID-19.
Fig. 4.
Cytokine storm induced by SARS-CoV-2. SARS-CoV-2 infect alveolar epithelial cells by penetration inside lung tissue leading to the recruitment of dendritic cells and macrophages differentiating into monocytes. Moreover, large quantities of proinflammatory cytokines and chemokines (IL6, IL8, IL10, IL12, TNF-a) are released leading to a big Cytokine Storm and causing tissue damage, with wide range of clinical manifestations (acute respiratory distress syndrome (ARDS), hyperinflammation, organs attack, pneumonia, and death of the infected patients. IL6: interleukin-6; IL8: interleukin-8; IL10: interleukin-10; IL12: interleukin-12; TGF- a: transforming growth factor- a.
2.3.5. MiRNA expression in human patients with COVID-19
Like intercellular miRNAs, circulating miRNAs or extracellular miRNAs play significant roles in gene regulation. These circulating miRNAs can be found and derived from blood origins like plasma, serum and several other biofluids including saliva, breast milk, tears, urine, peritoneal fluid, amniotic fluid, cerebrospinal fluid, seminal fluid etc. (O'Brien et al., 2018). These miRNAs have been extensively exploited in diagnosis, prognosis, and therapeutic guidance for several diseases (reviewed in (Guay and Regazzi, 2013, Shah et al., 2017, de Gonzalo-Calvo et al., 2019, van den Berg et al., 2020)). Some studies have also focused on evaluating the circulating miRNA profiles in COVID-19 patients. Tang et al. performed multi-transcriptome sequencing of noncoding RNAs including miRNAs obtained from the red blood cell depleted whole blood of moderate and severe COVID-19 patients (Tang et al., 2020a). Tang et al. showed several differentially expressed miRNAs based on the normal, moderate, and severe conditions of COVID-19 (Tang et al., 2020a). MiR-146a-5p, miR-21-5p, and miR-142-3p were consistently downregulated while miR-3605-3p was consistently upregulated in COVID-19 patients’ blood sample. Moreover, the expression of miR-15b-5p, miR-486-3p, and miR-486-5p were found to be upregulated, while the expression of miR-181a-2-3p, miR-31-5p, and miR-99a-5p were downregulated only in patients with severe COVID-19 (Tang et al., 2020a). The findings of Tang et al. indicate miRNAs as biomarkers of COVID-19 severity as well as their possible use as novel potential therapeutic targets for COVID-19 (Tang et al., 2020a).
Another study by Li et al. elucidated the differential expression of miRNAs in the peripheral blood from human patients with COVID-19 (Li et al., 2020a). They employed high-throughput sequencing to detect the differentially expressed miRNAs and found that 35 miRNAs were upregulated, and 38 miRNAs downregulated in the human COVID-19 patients as compared with healthy controls (Li et al., 2020a). Their findings indicated that miR-16-2-3p, miR-6501-5p, and miR-618 were upregulated in COVID-19 patients than in healthy controls while miR-183-5p, miR-627-5p, and miR-144-3p were downregulated in COVID-19 patients as compared to healthy controls (Li et al., 2020a). Thus, the findings by Tang et al. (Tang et al., 2020a) and Li et al. (Li et al., 2020a) demonstrate that circulating miRNAs could play a pivotal role in the diagnosis and treatment of COVID-19. However, a recent review by Pinilla et al. highlights methodological considerations, technical challenges and practice points related to the study of peripheral blood miRNAs in in COVID-19 patients (reviewed in (Pinilla et al., 2021)). Pinilla et al. (Pinilla et al., 2021) discussed several important points related to experimental design, data analysis and data interpretation that should be taken into account when evaluating the profiles of circulating miRNAs in COVID-19 patients (reviewed in (Pinilla et al., 2021)). Lukas et al. found that miR-200c was upregulated during disease progression in COVID-19 human patients and the expression correlated with the severity of disease (van de Sand et al., 2022). Another study demonstrated the involvement of miR-146a, miR-221, and miR-155 in inflammatory immune response in severe COVID-19 patients (Gaytán-Pacheco et al., 2022).
2.4. MicroRNA as therapeutic target against COVID-19: Possibilities and challenges
As discussed in previous sections (see above), miRNAs interact with SARS-CoV-2 either directly or indirectly via different mechanisms and therefore could be an important therapeutic target against COVID-19. There are already evidence that show that miRNA could be used as an effective antiviral agent. One example is that of miR-122 antagonist (Miravirsen) used in the treatment for hepatitis C virus (HCV) infection and is known to reduce viral RNA by sequestering miR-122 away from the viral genome, impeding the hepatitis C virus propagation (Jopling et al., 2005, Janssen et al., 2013). Antiviral miRNAs like miR-24, miR-124, and miR-744 have been reported to play significant role against respiratory virus infection via targeting p38 MAPK signaling pathways (McCaskill et al., 2017). A recent study by Mishra et al. demonstrated that miR-30e-5p increases innate immune responses by targeting negative regulators like SOCS1, SOCS3, ATG5, ATG12, TRIM38, TANK, and BECN1 of innate immune signalling pathways during hepatitis B virus (HBV) infection and systemic lupus erythematosus (Mishra et al., 2020).
Zhao et al. showed that miR-136 bind in the 3′ UTR of IL-6 and has antiviral effects against H5N1 influenza A virus, as well as vesicular stomatitis virus (VSV) in A549 human lung epithelial cells (Zhao et al., 2015). Chen et al. found that VSV infection led to an upregulation of miR-146a expression in mouse macrophages (Hou et al., 2009). In turn, miR-146a inhibits the production of the RIG-I-dependent antiviral response via feedback mechanism by targeting TRAF6 (TNFR-associated factor 6) and IRAK1/2 (interleukin 1 receptor-associated kinase 1/2) (Hou et al., 2009). Another study conducted by Liu et al. showed that VSV infection led to downregulation of miR-33/33* through the macrophage type I IFN receptor (IFNAR) (Liu et al., 2021). Furthermore, overexpression of miR-33/33* led to impaired RIG-I signaling, increasing viral load and lethality while attenuating type I IFN expression (Liu et al., 2021). In fact, miR-33/33* specifically prevented the formation of activated aggregates by inhibiting the activation of mitochondrial adaptor mitochondrial antiviral-signaling protein (MAVS) through adenosine monophosphate activated protein kinase (AMPK) (Liu et al., 2021). In brief, the findings by Liu et al. demonstrated miR-33/33* as negative modulators of the RNA virus-triggered innate immune response (Liu et al., 2021). In a different study, Yan et al. demonstrated the role of miR-221 in restricting human cytomegalovirus replication (Yan et al., 2019). miR-221 suppressed the expression of suppressor of cytokine signaling 1 (SOCS1) at both mRNA and protein levels (Yan et al., 2019). Moreover, miR-221 was shown to positively regulate the phosphorylation and activation of NF-κB via suppressing SOCS1 (Yan et al., 2019).
The use of miRNAs as a therapeutic target against virus does not end here. There are plenty more examples that could be cited for the use of miRNAs as antiviral agent (For details refer to reviews (Arghiani et al., 2021, Hu et al., 2021, Winkle et al., 2021, Ying et al., 2021, Panda et al., 2022, Paul et al., 2022)). Depending on the role of miRNA (either as enhancer or suppressor of viral replication), miRNA antagonists (such as antimiRs or antagomiRs) and miRNA mimics could be therapeutic approach against COVID-19 infections. Gasparello et al. discuss the use of miRNA mimics that directly target the 3′UTR of pro-inflammatory mRNAs to abate the COVID-19 “cytokine storm” (Gasparello et al., 2021). In one of the most recent publications, Beheshti and group elucidated the involvement of miR-2392 in SARS-CoV-2 infection (McDonald et al., 2021). miR-2392 is a circulating miRNA present in the blood and urine of COVID-19 patients and is directly involved in SARS-CoV-2 infection (McDonald et al., 2021). Mechanistically, miR-2392 suppresses the mitochondrial activity and its genes expression resulting in increased inflammation, glycolysis and hypoxia and several other symptoms that are closely associated with COVID-19 infection (McDonald et al., 2021). The group has also used a miRNA-based antiviral therapeutic strategy targeting miR-2392, using in vitro human and in vivo hamster models (McDonald et al., 2021). Of interest, inhibition of miR-2392 showed a significant reduction of SARS-CoV-2 viability in hamsters which could potentially put a check on COVID-19 in humans as well (McDonald et al., 2021).
3. Long non-coding RNAs (lncRNAs): An introduction
Long non-coding RNAs (lncRNAs) are over 200 nucleotides long transcripts that have wide range of regulatory functions (Arman and Moroy, 2020) ( Fig. 5 A). Most lncRNAs are generally transcribed by RNA polymerase II and are 5′ capped and polyadenylated at their 3′ ends (Guttman et al., 2009). It is well established that lncRNAs are involved in a myriad of physiological and biological processes and deregulation of these molecules is linked to development and progression of many pathophysiological health manifestations including cancer ( Fig. 5 B) (reviewed in (Gutschner and Diederichs, 2012)). With the employment of next-generation transcriptomic sequencing, tens of thousands of novel lncRNAs are being discovered at rapid pace outscoring the number of protein coding genes in human genome. It is interesting that lncRNAs depend on their expression pattern for the gene expression regulation (Jiang et al., 2016). Several lncRNAs, if not all, show tissue-specific or lineage-specific expression which makes them important candidates for therapeutic purposes. Moreover, the expression of majority of lncRNAs is much lower as compared to mRNAs (Statello et al., 2021).
Fig. 5.
Interaction between LncRNA and SARS-CoV-2. A) Classification of Long non-coding RNA (LncRNA) in the context of genomic locations and orientation. There are four major classes of LncRNA: 1: Intergenic LncRNA transcribed in the genomic region between two coding genes; 2: Bidirectional LncRNA transcribed from the opposite strand, in the opposite direction; 3: Antisense LncRNA transcribed from the opposite strand of coding genes and 4: Sense-overlapping LncRNA transcribed from the sense strand of an intronic region with no overlap of exonic sequence. B) Functions of LncRNA. LncRNA guide transcription factors to regulate genes expression, control translation rate, regulate RNA splicing, eRNA functions and chromatin remodeling and finally serve as sponge to titrate miRNA from their targets (Sequestration miRNA). C)) LncRNA targeting SARS-CoV-2 host IL6 and NLRP3 signaling and affecting NLRP3 inflammasome formation (such as LncRNA: NEAT1, ANRIL and GAS5) resulting in disruption of the cytokine storm.
Accumulating evidence suggest that lncRNAs have diverse regulatory functions in cells. Numerous lncRNAs affect transcription, which controls the expression of neighbouring genes. They also affect other aspects of chromatin biology, including DNA replication and the response to and repair of DNA damage. Other lncRNAs may have structural or regulatory functions that affect signaling pathways and various mRNA life cycles, including splicing, turnover, and translation. As a result, lncRNAs have a significant physiological impact on a range of cellular activities, and diseases that alter their expression are common. The different expression patterns of these functional lncRNAs have the potential to be used as best disease biomarkers, and strategies are being made for their therapeutic targeting (Statello et al., 2021).
A growing body of evidence also suggests that lncRNAs have critical regulatory functions in viral infections and during the antiviral immune response (reviewed in (Liu and Ding, 2017, Wang, 2018)). LncRNAs have been well described for their role as regulators of viral infection and the interferon antiviral response (reviewed in (Qiu et al., 2018)). Expression of cellular lncRNAs is dysregulated during viral infections, ultimately regulating multiple host processes and facilitating the progression of viral infection (Yi et al., 2019). Given the importance of lncRNAs in viral infections, we discuss their role in COVID-19 or SARS-CoV-2 infections ( Table 2 ).
Table 2.
LncRNAs associated with SARS-CoV-2 infections.
| LncRNA | Regulation | Expression | Reference |
|---|---|---|---|
|
MALAT1 NEAT1 DANCR TUG1 |
Regulation of inflammation |
Upregulated Upregulated Downregulated Upregulated |
(Dai et al., 2018, Tian et al., 2018, Hao et al., 2020) (Meydan et al., 2020, Vishnubalaji et al., 2020, Rodrigues et al., 2021) (Meydan et al., 2020) (Liang and Ren, 2018) |
|
ANRIL GAS5 |
Regulation of inflammasome responses |
Upregulated |
(Hu et al., 2019) (Li et al., 2018a) |
| HAND2-AS1 | Regulation of IL-6 | Upregulated | (Si et al., 2021) |
|
MIR3142HG AC048341.2 LINC02015 INHBA-AS1 AC008760.2 AL109615.3 C1RL-AS1 AC006058.1 MEG3 |
Role in cell survival and regulation of gene expression |
Upregulated |
(Turjya et al., 2020) |
|
SNHG8 UGDH-AS1 AC007743.1 KCNQ1OT1 LINC00504 AC087473.1 GAS6-AS1 AP000542.3 AL603839.3 FAM230J LINC00937 FAM106A KCNQ1OT1 |
Role in cell survival and regulation of gene expression |
Downregulated |
(Turjya et al., 2020) |
3.1. LncRNAs and coronaviruses
Although associations between lncRNAs and other types of coronaviruses have been poorly reported, several studies have reported significant associations between them. Peng et al. (Peng et al., 2010) were the first to describe an association between coronavirus infection and lncRNAs. Particularly, they have showed hundreds of differentially expressed lncRNAs in response to SARS-CoV-1 infection which could aberrantly modulate host responses together with the innate immune signaling (Peng et al., 2010). Porcine epidemic diarrhea virus (PEDV), which belongs to the Alphacoronavirus genus within the Coronaviridae family and is responsible for the Porcine epidemic diarrhea (PED), has also been reported to be associated with the modulation of the host lncRNA expression. Specifically, PEDV infections were linked to lncRNAs responsible for immune activation in the ileum (Chen et al., 2019). Differential expression of lncRNAs were also reported during Porcine deltacoronavirus (PDCoV) infection, which is associated with the intestinal necrosis of piglets, thinning of the intestinal wall and severe villus atrophy in the small intestine (Tang et al., 2019). Of the 101 lncRNAs, 75 were upregulated and 26 downregulated in Avian infectious bronchitis virus (IBV)-stimulated avian dendritic cells (Lin et al., 2019). Moreover, lncRNA TCONS_00058367 were identified to be differentially expressed in Transmissible gastroenteritis virus (TGEV)-infected porcine intestinal epithelial cell-jejunum 2 cells (Ma et al., 2019). It has also been reported that porcine delta coronavirus infection is associated with differential expression of lncRNAs in Swine testicular cells and porcine jejunum intestinal epithelial cells, and the differentially expressed lncRNAs were found to be associated with metabolism and TNF signaling (Liu et al., 2019).
3.2. LncRNAs with interferon and inflammatory response to SARS-CoV-2: A cytokine storm modulation
LncRNAs have been shown to play pivotal role in inflammatory disease progression (reviewed in (Pearson and Jones, 2016, Mathy and Chen, 2017). Peng at al. (Peng et al., 2010) employed next-generation sequencing to perform whole transcriptome analysis of the host response to severe SARS-CoV infection across four founder mouse strains of the Collaborative Cross (CC), a recombinant inbred mouse resource for mapping complex traits (Churchill et al., 2004). Peng at al. showed about 500 lncRNAs with widespread differential expression in response to virus infection and suggested that lncRNAs play important role in innate immune responses via signal transducer and activator of transcription 1 (STAT1) (Peng et al., 2010). Here, we discuss lncRNAs with interferon and inflammatory response to SARS-CoV-2.
3.2.1. LncRNAs MALAT1, NEAT1, DANCR as promising lncRNAs against SARS-CoV-2
Alajez and group (Vishnubalaji et al., 2020) carried out a deep and robust transcriptome analysis of already published data by Blanco-Melo et al. (Blanco-Melo et al., 2020) from lung biopsies derived from COVID-19 patients as well as from primary normal human bronchial epithelial cells (NHBE) during the SARS-CoV-2 infection (Vishnubalaji et al., 2020). The findings of Alajez and group revealed that SARS-CoV-2 infection led to the activation of interferon (IFN) and inflammatory response (Vishnubalaji et al., 2020). The group also identified 155 upregulated and 195 downregulated lncRNAs in response to SARS-CoV-2 viral infection (Vishnubalaji et al., 2020). Among the upregulated lncRNAs, few well known included MALAT1, NEAT1, SNHG25 while some downregulated lncRNAs were DANCR, SNHG7, HOTAIRM1 (Vishnubalaji et al., 2020). They (Vishnubalaji et al., 2020) also compared lncRNAs in between SARS-CoV-2 NHBE and COVID-19 patient derived lung tissue and identified 5 common upregulated lncRNAs such as SNHG25, RCC2-AS1 etc. and 57 common downregulated lncRNAs such as SNHG19, SNHG7 etc. These common upregulated or downregulated lncRNAs could possibly be used as biomarkers and therapeutic targets during SARS-CoV-2 infection (Vishnubalaji et al., 2020). However, their specific roles in response to SARS-CoV-2 infection remains to be elucidated.
Meydan et al. studied the role of lncRNAs in the contribution of inflammatory response to COVID-19. The group carried out both bioinformatic and in vitro studies for their research work. They used the already available data including cohort of Genome Tissue expression (GTEx) project (https://www.gtexportal.org/) and exploited RNA-seq datasets on lung, brain and blood of healthy men and women across ages. They also did the analysis of RNA-seq datasets of SARS-CoV-2 infected lung cells and checked the expression of miRNAs and lncRNAs (Blanco-Melo et al., 2020, Meydan et al., 2020). They revealed that lncRNA DANCR and NEAT1 were downregulated in lung tissues of inflammation prone individuals and the expression of DANCR was inversely correlated with inflammation-controlling transcripts in SARS-COV-2. Moreover, it was observed that the expression levels of both DANCR and NEAT1 were inversely corelated with age and sex of individuals included from GTEx lung RNA-seq dataset. Both NEAT1 and DANCR were downregulated in SARS-CoV-2 infected cells as compared to non-infected bronchial epithelial cells and lung adenocarcinoma cells (A549). However, DANCR was the most prominent downregulated lncRNA among the list. Mechanistically they concluded that both DANCR and NEAT1 could impede inflammation via sponging miRNAs, or by modulating transcription factors like STAT3 which is responsible for controlling inflammatory responses. The findings by Meydan et al. highlight the role of DANCR and NEAT1 in inflammation and their plausible roles in COVID-19 as well (Meydan et al., 2020). The downregulation of NEAT1 in SARS-CoV-2 infected cells as compared to non-infected bronchial epithelial cells in this study is controversial to the findings of Alajez and group (Vishnubalaji et al., 2020). Using the same RNA-seq data from a recent study by Blanco-Melo et al. (Blanco-Melo et al., 2020), the groups of Meydan et al. (Meydan et al., 2020) and Vishnubalaji et al. (Vishnubalaji et al., 2020) found different expression pattern for NEAT1. Vishnubalaji et al. (Vishnubalaji et al., 2020) found NEAT1 to be upregulated in response to SARS-CoV-2 viral infection while Meydan et al. (Meydan et al., 2020) found the opposite. However, Meydan et al. included several datasets as well as in vitro experiments to support their findings. In a very recent study, single-cell transcriptome data of female patients with either systemic lupus erythematosus or COVID-19 showed dysregulation of lncRNA XIST (Yu et al., 2021). It was revealed that XIST-dependent genes escaped during COVID-19 infection, specifically in CD11c+ atypical memory B cells (ABCs), suggesting role of XIST in females with SARS-CoV-2 viral infection (Yu et al., 2021). One of the recent studies (Rodrigues et al., 2021) showed that MALAT1 and NEAT1 were highly expressed in saliva and nasopharyngeal swab sample of human COVID-19 patients (n = 34) as compared to healthy individuals (n = 46) (Rodrigues et al., 2021). MALAT1 and NEAT1 were found to be significantly expressed mainly in nasopharyngeal and saliva samples, respectively (Rodrigues et al., 2021). In brief, the findings by Oliveira and group indicate the importance of MALAT1 and NEAT1 which could plausibly be used as biomarker or targets for COVID-19 therapy (Rodrigues et al., 2021). Recently Huang et al. used human COVID-19 patient samples for single cell transcriptomic analysis in bronchoalveolar lavage (BAL) cells and peripheral blood mononuclear cells (PBMCs). They discovered NEAT1 and MALAT1 as highly differentially expressed lncRNA between mild and severe COVID-19 patients indicating NEAT1 and MALAT1 as potential components of immune dysregulation in COVID-19 (Huang et al., 2022).
3.2.2. Involvement of NEAT1, ANRIL, GAS5 in inflammasome formation leading to cytokine storm
NOD-like receptor protein 3 (NLRP3) inflammasome as well as interleukin-6 (IL-6) are known to be accountable for inflammatory cytokine storm and are therefore pathologically associated with patients infected with COVID-19 (reviewed in (Tay et al., 2020, Tufan et al., 2020)). It is not surprising that lncRNAs have been shown to regulate NLRP3 inflammasome and IL-6-assocciated inflammatory signaling pathways (Yi et al., 2017, Yu et al., 2018, Zhang and Chu, 2019, Paniri and Akhavan-Niaki, 2020) ( Fig. 5 C). Well known lncRNAs like NEAT1, ANRIL, GAS5 have been shown to be involved in inflammasome formation. Zhang et al. showed that Neat1 associated and activated NLRP3 inflammasome in mouse macrophages (Zhang et al., 2019). LPS induced murine immortalized bone marrow-derived macrophages (iBMDMs) facilitated the translocation of Neat1 from nucleus to the cytoplasm in order to modulate inflammasome activation which eventually activated caspase-1 and led to the release of interleukin-1β (Zhang et al., 2019). The inflammatory responses were greatly reduced in Neat1 knock out mouse models of peritonitis and pneumonia, highlighting the inflammatory role of Neat1 (Zhang et al., 2019). In a recent study involving human COVID-19 samples, NEAT1 was found to be highly expressed in COVID-19 patients as compared to controls. Moreover, NEAT1 showed significant correlations with the measured cytokines IL-6 (Tayel et al., 2022). Antisense non-coding RNA in the INK4 locus (ANRIL) acts as a competing endogenous RNA (ceRNA) in order to affect inflammasome responses in uric acid nephropathy patients (Hu et al., 2019). Mechanistically, it was demonstrated that ANRIL sponged miR-122-5p in order to upregulate the expression of BRCA1-BRCA2-containing complex subunit 3 (BRCC3) and NLRP3 inflammasome to exert the pathogenic effect in uric acid nephropathy (Hu et al., 2019). LncRNA growth arrest-specific transcript 5 (GAS5) was found to act as tumor suppressor in ovarian cancer (Li et al., 2018a). The overexpression of GAS5 significantly repressed ovarian cancer by inducing inflammasome formation which further increased IL-1β secretion (Li et al., 2018a). The in vitro experiments involving the knockdown of GAS5 revealed the tumor suppressive activity of GAS5 which is mediated through inflammasome-induced inflammatory cytokine release (Li et al., 2018a). LncRNAs also play key roles in the regulation of IL-6 (reviewed in (Zhang and Chu, 2019)). LncRNA GAS5 was found to be highly expressed in polycystic ovary syndrome (PCOS) and acted as an oncogene (Wang et al., 2020). Overexpression of GAS5 curtailed cell apoptosis in granulosa-like tumor cell line (KGN) promoting PCOS (Wang et al., 2020). More importantly, the expression levels of both GAS5 and IL-6 were positively correlated indicating the implication of GAS5 and IL-6 in PCOS (Wang et al., 2020). The role of a relatively new lncRNA HAND2-AS1 in the regulation of IL-6 in human chondrocytes has been recently revealed (Si et al., 2021). It was noticed that the expression of HAND2-AS1 and IL-6 were dysregulated in plasma and synovial fluid of osteoarthritis patients (Si et al., 2021). The ectopic expression of HAND2-AS1 led to the inhibition of IL-6 expression in chondrocytes, while the treatment with exogenous IL-6 had no effect on the expression of HAND2-AS1 indicating a direct regulation of IL-6 by HAND2-AS1 in human chondrocytes (Si et al., 2021). MALAT1 has a dual role regarding inflammation responses particularly with the regulation of IL-6. MALAT1 was highly expressed in acute kidney injury with likelihood that MALAT1 might have an anti-inflammatory role (Tian et al., 2018). Moreover, ablation of MALAT1 in cobalt chloride treated HK2 cells led to HIF-1α and NF-κB activation which eventually upregulated the concentrations of inflammatory cytokines like IL-6 and TNF-ɑ, indicating an anti-inflammatory role of MALAT1 in acute kidney injury (Tian et al., 2018). On the other hand, the inflammatory role of MALAT1 is revealed in LPS-induced acute lung injury (Dai et al., 2018). MALAT1 acted as a ceRNA for miR-146a and negatively regulated the expression of miR-146a (Dai et al., 2018). The knockdown of MALAT1 showed the suppression of inflammatory responses via an increase in miR-146a expression in LPS-induced acute lung injury (Dai et al., 2018).
3.2.3. Regulation of lncRNAs by IL-6 during lung infection
There are certain research works which show that IL-6 also regulates lncRNAs. Hao et al. revealed the role of HPV18 E6/E7 and IL-6/STAT3 in the regulation of MALAT1 in cervical cancer cells (Hao et al., 2020). It was found that both HPV18 E6/E7 and IL-6/STAT3 reciprocally regulated each other in HeLa cells (Hao et al., 2020). Moreover, STAT3 bound to the enhancer region of MALAT1 while HPV18 E6/E7 as well as IL-6/STAT3 synergistically upregulated the expression of MALAT1 in cervical cancer cells (Hao et al., 2020). A different study highlighted the role of IL-6 in the stimulation of lncRNA ZEB2-AS1 in non-small cell lung cancer (Chen et al., 2020a). Chromatin Immunoprecipitation (ChIP) assay verified the interaction between STAT1 and lncRNA ZEB2-AS1 in lung cancer cells (Chen et al., 2020a). The expression of lncRNA ZEB2-AS1 increased in dose-dependent manner in IL-6-treated A549 cells (Chen et al., 2020a). In brief, the study by Chen et al. showed the stimulation of lncRNA ZEB2-AS1 by IL-6 which further worsens non-small cell lung cancer through activating STAT1 (Chen et al., 2020a).
3.3. LncRNAs differentially expressed in the peripheral blood of human COVID-19 patients
Wu et al. (Wu et al., 2021) collected blood samples from three recurrent COVID-19 patients and three healthy individuals and sequenced circular RNA (circRNA), lncRNA and mRNA via microarray in order to highlight the differentially expressed genes from each category (Wu et al., 2021). They also used exosomes related databases to find lncRNAs associated with exosomes. Exosomes are small membrane vesicles (30–150 nm) which generally contain RNA, proteins and are involved in immune response, antigen presentation, migration, differentiation, tumor invasion (Dou et al., 2021, Ju et al., 2021, Wu et al., 2021). Finally, they compared their findings to exosomes related databases and found 10 differentially expressed lncRNA related to exosomes (Wu et al., 2021). Few of the upregulated lncRNAs found in the COVID-19 patients were ENST00000504735 (gene symbol: AC016933.1), NR_037411.1 (gene symbol: MIR3617), ENST00000577988 (gene symbol: SNORD3B-1), ENST00000527803 (gene symbol: AP001922.5), ENST00000391111 (gene symbol: RNU11-3P) (Wu et al., 2021). Wang and group (Wu et al., 2021) also performed the GO and KEGG enrichment analysis of the differentially expressed lncRNA and were primarily found to be involved in the regulation of cell cycle, apoptosis, immune inflammation, signaling pathways. The authors eventually conclude that the differentially expressed lncRNAs could be further used as biomarker and treating COVID-19 (Wu et al., 2021). However, their findings need to be validated initially for their target efficacy using in vitro and in vivo models before implementing them to be used as biomarker or in the treatment of COVID-19.
3.4. LncRNAs in SARS-CoV-2 infected lung epithelial cells
In a study conducted by Islam and group (Turjya et al., 2020), differentially expressed lncRNAs were extracted from RNA-seq dataset of SARS-CoV-2 infected lung epithelial cells. Consequently, they identified 21 differentially expressed lncRNAs which were having prominent roles in cell survival and regulation of gene expression (Turjya et al., 2020). Among the 21 differentially expressed lncRNAs, 9 lncRNAs were upregulated while 12 were found to be downregulated. The upregulated lncRNAs consisted of MIR3142HG, AC048341.2, LINC02015, INHBA-AS1, AC008760.2, AL109615.3, C1RL-AS1, AC006058.1 and MEG3 while the downregulated lncRNAs comprised of SNHG8, UGDH-AS1, AC007743.1, KCNQ1OT1, LINC00504, AC087473.1, GAS6-AS1, AP000542.3, AL603839.3, FAM230J, LINC00937 and FAM106A (Turjya et al., 2020). Furthermore, these differentially expressed lncRNAs were found to interact via RNA-RNA interactions and RNA–protein interactions with four and two differentially expressed protein-coding genes, respectively (Turjya et al., 2020). The protein coding gene ADAR interacted with lncRNAs KCNQ1OT1, GAS6-AS1, MEG3 and INHBA-AS1 (Turjya et al., 2020). The other protein coding gene YWHAG interacted with KCNQ1OT1, SNHG8 and GAS6-AS1. Both ADAR and YWHAG interacted with the lncRNAs via RNA–protein interactions (Turjya et al., 2020). Protein coding genes like EDN1, KYNU, MALL, TLR2 interacted via RNA-RNA interactions with lncRNAs UGDH-AS1, FAM230J, AC006058.1 and KCNQ1OT1, respectively (Turjya et al., 2020). In addition, the above mentioned differentially expressed lncRNAs were found to communicate with few host genes which interacted with SARS-CoV-2 proteins like M (Membrane), N (Nucleocapsid), Nsp8, Nsp12, Nsp13, and ORF8 (Turjya et al., 2020). Last but not the least, the identified lncRNAs were found to block or inhibit the suppressive effect of some microRNAs like miRNA let-7c, miRNA let-7f, miR-185-5p, miR-197-5p, miR-200c-5p etc. which are found to be induced in viral infections (Turjya et al., 2020). In brief, Islam and group highlight the roles (cell survival, viral replication, and immune defense) of lncRNAs during SARS-CoV-2 infection, making these lncRNAs as good candidates for therapeutic targets against COVID-19.
3.5. Targeting lncRNAs as diagnostic marker and therapeutic agent
LncRNAs are well known candidates that could be used as diagnostic marker and therapeutic targets in different types of cancer (Arun et al., 2018). LncRNA DANCR act as a diagnostic marker in hepatocellular carcinoma (Ma et al., 2016). Circulating lncRNAs like XLOC_009167 and LINC00152 have been shown to be diagnostic markers in lung cancer (Jiang et al., 2018) and hepatocellular carcinoma (Yuan et al., 2017), respectively. There are plenty of other lncRNAs that have been shown or used as diagnostic markers in different types of cancer (Shi et al., 2016, Bolha et al., 2017, Quan et al., 2018, Elias-Rizk et al., 2020, Xiao et al., 2020). The possible use of lncRNAs as diagnostic marker in COVID-19 is also coming into light. Cheng J et al. observed that lncRNA GATA5 was significantly upregulated in severe condition patients as compared to healthy controls, indicating a positive correlation of GATA5 with COVID-19 severity (Cheng et al., 2021). As described previously, Meydan et al. revealed that NEAT1 and DANCR were downregulated in SARS-CoV-2 infected cells as compared to non-infected bronchial epithelial cells (NHBE) and the expression of lncRNA DANCR was inversely correlated with inflammation-controlling genes in SARS-COV-2 (Meydan et al., 2020). Moreover, Oliveira and his group demonstrated that MALAT1 and NEAT1 are good biomarker candidates for COVID-19, as they were upregulated in human saliva and nasopharyngeal swab patient samples as compared to healthy counterparts (Rodrigues et al., 2021). These findings indicate that the expression of lncRNAs GATA5, MALAT1, NEAT1 and DANCR could be used as promising diagnostic markers and eventually as therapeutic targets against COVID-19. Table 3 summarizes some of these diagnostic biomarkers during SARS-CoV-2 infection.
Table 3.
Potential diagnostic biomarkers associated with SARS-CoV-2 infection.
| LncRNA | Regulation | Expression | Reference |
|---|---|---|---|
|
SNHG7 HOTAIRM1 RCC2-AS1 SNHG19 XIST AC016933.1 MIR3617 SNORD3B-1 AP001922.5 RNU11-3P XLOC_009167 LINC00152 GATA5 |
Biomarker during SARS-CoV-2 infection |
Downregulated Downregulated Upregulated Downregulated Downregulated Upregulated Upregulated Upregulated Upregulated Upregulated Upregulated Upregulated Upregulated |
(Vishnubalaji et al., 2020) (Vishnubalaji et al., 2020) (Vishnubalaji et al., 2020) (Vishnubalaji et al., 2020) (Yu et al., 2021) (Wu et al., 2021) (Wu et al., 2021) (Wu et al., 2021) (Wu et al., 2021) (Wu et al., 2021) (Jiang et al., 2018) (Jiang et al., 2018) (Cheng et al., 2021) |
Majority of lncRNAs exhibit their expression in a highly tissue- and cell-type specific manner (Cabili et al., 2011, Fatica and Bozzoni, 2014) making them attractive and efficacious targets against cancer as well as viral diseases like COVID-19. The therapeutic potential of lncRNAs in cancer and against viruses have been well elaborated by different groups (reviewed in (Ding et al., 2016, Fortes and Morris, 2016, Arun et al., 2018). As we discussed above (in section 3.1 and its sub-sections), abnormal expression of lncRNAs in COVID-19 lead to inflammatory response leading to cytokine storm. Therefore, lncRNAs could downregulate the cytokine expression, improving the proinflammatory immune response to COVID-19 infection and hence curtailing the undesirable cytokine storms. LncRNAs like NEAT1, ANRIL, GAS5 have been shown to be involved in inflammasome formation via regulating NLRP3 inflammasome and IL-6-assocciated inflammatory signaling pathways (Paniri and Akhavan-Niaki, 2020). Therefore, it raises the possibility of targeting these lncRNAs to shut down their inflammatory response and to combat COVID-19. An anti-inflammatory agent ‘Emodin’ has been shown to exert its effect via recruiting lncRNA Tug1 (Liang and Ren, 2018). In fact, Emodin mitigates lipopolysaccharide (LPS) induced cell death (apoptosis) and inflammation by upregulating lncRNA Tug1 in murine chondrogenic ATDC5 cells, thereby blocking Notch and NF-κB pathways (Liang and Ren, 2018). Wei et al. showed that the knockdown of MALAT1 suppresses inflammatory injury by downregulating IL-8 and inhibiting immune cell infiltration (Wei et al., 2019). In this paragraph we discuss only few but not all the lncRNAs that could possibly be targeted against COVID-19. And the list of candidate lncRNAs go on piling up. We thus conclude that lncRNAs show potential as targets for therapeutics in SARS-CoV-2 pathogenesis. However, their use as therapeutic target against COVID-19 in human needs further study using different mouse models.
Declarations.
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Availability of data and material: Not applicable.
Funding: The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Authors' contributions: KA has generated the text, ZD has generated figures and tables, EB edited the manuscript for final submission. All authors contributed to the article and approved the submitted version.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Not applicable.
Edited by Yavuz Dodurga
Data availability
No data was used for the research described in the article.
References
- Abu-Izneid T., AlHajri N., Mohammed Ibrahim A., Noushad Javed M., Mustafa Salem K., Hyder Pottoo F., Amjad Kamal M. Micro-RNAs in the regulation of immune response against SARS COV-2 and other viral infections. J. Adv. Res. 2020 doi: 10.1016/j.jare.2020.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu-Izneid T., AlHajri N., Ibrahim A.M., Javed M.N., Salem K.M., Pottoo F.H., Kamal M.A. Micro-RNAs in the regulation of immune response against SARS CoV-2 and other viral infections. J. Adv. Res. 2021;30:133–145. doi: 10.1016/j.jare.2020.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F., Vanstapel A., Werlein C., Stark H., Tzankov A., Li W.W., Li V.W., Mentzer S.J., Jonigk D. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N. Engl. J. Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghagoli, G., Gallo Marin, B., Katchur, N.J., Chaves-Sell, F., Asaad, W.F. and Murphy, S.A., 2020. Neurological Involvement in COVID-19 and Potential Mechanisms: A Review. Neurocrit Care. [DOI] [PMC free article] [PubMed]
- Al-Rawi N.H., Al-Marzooq F., Al-Nuaimi A.S., Hachim M.Y., Hamoudi R. Salivary microRNA 155, 146a/b and 203: A pilot study for potentially non-invasive diagnostic biomarkers of periodontitis and diabetes mellitus. PLoS One. 2020;15:e0237004. doi: 10.1371/journal.pone.0237004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arghiani N., Nissan T., Matin M.M. Role of microRNAs in COVID-19 with implications for therapeutics. Biomed. Pharmacother. 2021;144 doi: 10.1016/j.biopha.2021.112247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arisan E.D., Dart A., Grant G.H., Arisan S., Cuhadaroglu S., Lange S., Uysal-Onganer P. The Prediction of miRNAs in SARS-CoV-2 Genomes: hsa-miR Databases Identify 7 Key miRs Linked to Host Responses and Virus Pathogenicity-Related KEGG Pathways Significant for Comorbidities. Viruses. 2020;12 doi: 10.3390/v12060614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arman K., Moroy T. Crosstalk Between MYC and lncRNAs in Hematological Malignancies. Front. Oncol. 2020;10 doi: 10.3389/fonc.2020.579940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arun G., Diermeier S.D., Spector D.L. Therapeutic Targeting of Long Non-Coding RNAs in Cancer. Trends Mol. Med. 2018;24:257–277. doi: 10.1016/j.molmed.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashour H.M., Elkhatib W.F., Rahman M.M., Elshabrawy H.A. Insights into the Recent 2019 Novel Coronavirus (SARS-CoV-2) in Light of Past Human Coronavirus Outbreaks. Pathogens. 2020;9 doi: 10.3390/pathogens9030186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badry A., Jaspers V.L.B., Waugh C.A. Environmental pollutants modulate RNA and DNA virus-activated miRNA-155 expression and innate immune system responses: Insights into new immunomodulative mechanisms. J. Immunotoxicol. 2020;17:86–93. doi: 10.1080/1547691X.2020.1740838. [DOI] [PubMed] [Google Scholar]
- Bandopadhyay M., Bharadwaj M. Exosomal miRNAs in hepatitis B virus related liver disease: a new hope for biomarker. Gut Pathog. 2020;12:23. doi: 10.1186/s13099-020-00353-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel D.P. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeraka N.M., Tulimilli S.V., Karnik M., Sadhu S.P., Pragada R.R., Aliev G., Madhunapantula S.V. The Current Status and Challenges in the Development of Vaccines and Drugs against Severe Acute Respiratory Syndrome-Corona Virus-2 (SARS-CoV-2) Biomed Res. Int. 2021;2021:8160860. doi: 10.1155/2021/8160860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belouzard S., Millet J.K., Licitra B.N., Whittaker G.R. Mechanisms of coronavirus cell entry mediated by the viral spike protein. Viruses. 2012;4:1011–1033. doi: 10.3390/v4061011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt K., Kato M., Natarajan R. Mini-review: emerging roles of microRNAs in the pathophysiology of renal diseases. Am. J. Physiol. Renal Physiol. 2016;310:F109–F118. doi: 10.1152/ajprenal.00387.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch C.J., Clothier H.J., Seccull A., Tran T., Catton M.C., Lambert S.B., Druce J.D. Human coronavirus OC43 causes influenza-like illness in residents and staff of aged-care facilities in Melbourne, Australia. Epidemiol. Infect. 2005;133:273–277. doi: 10.1017/s0950268804003346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco-Melo D., Nilsson-Payant B.E., Liu W.C., Uhl S., Hoagland D., Moller R., Jordan T.X., Oishi K., Panis M., Sachs D., Wang T.T., Schwartz R.E., Lim J.K., Albrecht R.A., tenOever B.R. Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19. Cell. 2020;181(1036–1045):e9. doi: 10.1016/j.cell.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolha L., Ravnik-Glavac M., Glavac D. Long Noncoding RNAs as Biomarkers in Cancer. Dis. Markers. 2017;2017:7243968. doi: 10.1155/2017/7243968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozgeyik I. Therapeutic potential of miRNAs targeting SARS-CoV-2 host cell receptor ACE2. Meta Gene. 2021;27 doi: 10.1016/j.mgene.2020.100831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabili M.N., Trapnell C., Goff L., Koziol M., Tazon-Vega B., Regev A., Rinn J.L. Integrative annotation of human large intergenic noncoding RNAs reveals global properties and specific subclasses. Genes Dev. 2011;25:1915–1927. doi: 10.1101/gad.17446611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderon-Dominguez M., Trejo-Gutierrez E., González-Rovira A., Beltrán-Camacho L., Rojas-Torres M., Eslava-Alcón S., Sanchez-Morillo D., Calderon-Dominguez J., Martinez-Nicolás M.P., Gonzalez-Beitia E., Nieto-Martín M.D., Trujillo-Soto T., Rodríguez-Iglesias M.A., Moreno J.A., Moreno-Luna R., Durán-Ruiz M.C. Serum microRNAs targeting ACE2 and RAB14 genes distinguish asymptomatic from critical COVID-19 patients. Mol. Ther. Nucleic Acids. 2022;29:76–87. doi: 10.1016/j.omtn.2022.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Guo Y., Pan Y., Zhao Z.J. Structure analysis of the receptor binding of 2019-nCoV. Biochem. Biophys. Res. Commun. 2020 doi: 10.1016/j.bbrc.2020.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T., Li J., Zhou M.H., Xu L.J., Pan T.C. IL-6 stimulates lncRNA ZEB2-AS1 to aggravate the progression of non-small cell lung cancer through activating STAT1. Eur. Rev. Med. Pharmacol. Sci. 2020;24:3734–3740. doi: 10.26355/eurrev_202004_20837. [DOI] [PubMed] [Google Scholar]
- Chen Y., Wang X. miRDB: an online database for prediction of functional microRNA targets. Nucleic Acids Res. 2020;48:D127–D131. doi: 10.1093/nar/gkz757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Zhang C., Zhang N., Liu G. Porcine endemic diarrhea virus infection regulates long noncoding RNA expression. Virology. 2019;527:89–97. doi: 10.1016/j.virol.2018.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J., Zhou X., Feng W., Jia M., Zhang X., An T., Luan M., Pan Y., Zhang S., Zhou Z., Wen L., Sun Y., Zhou C. Risk stratification by long non-coding RNAs profiling in COVID-19 patients. J. Cell Mol. Med. 2021;25:4753–4764. doi: 10.1111/jcmm.16444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill G.A., Airey D.C., Allayee H., Angel J.M., Attie A.D., Beatty J., Beavis W.D., Belknap J.K., Bennett B., Berrettini W., Bleich A., Bogue M., Broman K.W., Buck K.J., Buckler E., Burmeister M., Chesler E.J., Cheverud J.M., Clapcote S., Cook M.N., Cox R.D., Crabbe J.C., Crusio W.E., Darvasi A., Deschepper C.F., Doerge R.W., Farber C.R., Forejt J., Gaile D., Garlow S.J., Geiger H., Gershenfeld H., Gordon T., Gu J., Gu W., de Haan G., Hayes N.L., Heller C., Himmelbauer H., Hitzemann R., Hunter K., Hsu H.C., Iraqi F.A., Ivandic B., Jacob H.J., Jansen R.C., Jepsen K.J., Johnson D.K., Johnson T.E., Kempermann G., Kendziorski C., Kotb M., Kooy R.F., Llamas B., Lammert F., Lassalle J.M., Lowenstein P.R., Lu L., Lusis A., Manly K.F., Marcucio R., Matthews D., Medrano J.F., Miller D.R., Mittleman G., Mock B.A., Mogil J.S., Montagutelli X., Morahan G., Morris D.G., Mott R., Nadeau J.H., Nagase H., Nowakowski R.S., O'Hara B.F., Osadchuk A.V., Page G.P., Paigen B., Paigen K., Palmer A.A., Pan H.J., Peltonen-Palotie L., Peirce J., Pomp D., Pravenec M., Prows D.R., Qi Z., Reeves R.H., Roder J., Rosen G.D., Schadt E.E., Schalkwyk L.C., Seltzer Z., Shimomura K., Shou S., Sillanpaa M.J., Siracusa L.D., Snoeck H.W., Spearow J.L., Svenson K., et al. The Collaborative Cross, a community resource for the genetic analysis of complex traits. Nat. Genet. 2004;36:1133–1137. doi: 10.1038/ng1104-1133. [DOI] [PubMed] [Google Scholar]
- Dai L., Zhang G., Cheng Z., Wang X., Jia L., Jing X., Wang H., Zhang R., Liu M., Jiang T., Yang Y., Yang M. Knockdown of LncRNA MALAT1 contributes to the suppression of inflammatory responses by up-regulating miR-146a in LPS-induced acute lung injury. Connect. Tissue Res. 2018;59:581–592. doi: 10.1080/03008207.2018.1439480. [DOI] [PubMed] [Google Scholar]
- de Gonzalo-Calvo D., Vea A., Bar C., Fiedler J., Couch L.S., Brotons C., Llorente-Cortes V., Thum T. Circulating non-coding RNAs in biomarker-guided cardiovascular therapy: a novel tool for personalized medicine? Eur. Heart J. 2019;40:1643–1650. doi: 10.1093/eurheartj/ehy234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desjarlais M., Wirth M., Lahaie I., Ruknudin P., Hardy P., Rivard A., Chemtob S. Nutraceutical Targeting of Inflammation-Modulating microRNAs in Severe Forms of COVID-19: A Novel Approach to Prevent the Cytokine Storm. Front. Pharmacol. 2020;11 doi: 10.3389/fphar.2020.602999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devarakonda C.K.V., Meredith E., Ghosh M., Shapiro L.H. Coronavirus Receptors as Immune Modulators. J. Immunol. 2021;206:923–929. doi: 10.4049/jimmunol.2001062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickey L.L., Hanley T.M., Huffaker T.B., Ramstead A.G., O'Connell R.M., Lane T.E. MicroRNA 155 and viral-induced neuroinflammation. J. Neuroimmunol. 2017;308:17–24. doi: 10.1016/j.jneuroim.2017.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y.Z., Zhang Z.W., Liu Y.L., Shi C.X., Zhang J., Zhang Y.G. Relationship of long noncoding RNA and viruses. Genomics. 2016;107:150–154. doi: 10.1016/j.ygeno.2016.01.007. [DOI] [PubMed] [Google Scholar]
- Dou R., Zhang X., Xu X., Wang P., Yan B. Mesenchymal stem cell exosomal tsRNA-21109 alleviate systemic lupus erythematosus by inhibiting macrophage M1 polarization. Mol. Immunol. 2021;139:106–114. doi: 10.1016/j.molimm.2021.08.015. [DOI] [PubMed] [Google Scholar]
- Durvasula R.V., Shankland S.J. Activation of a local renin angiotensin system in podocytes by glucose. Am. J. Physiol. Renal Physiol. 2008;294:F830–F839. doi: 10.1152/ajprenal.00266.2007. [DOI] [PubMed] [Google Scholar]
- Elemam, N.M., Hasswan, H., Aljaibeji, H., Sharif-Askari, N.S., Halwani, R., Taneera, J. and Sulaiman, N., 2022. Profiling Levels of Serum microRNAs and Soluble ACE2 in COVID-19 Patients. Life (Basel) 12. [DOI] [PMC free article] [PubMed]
- Elias-Rizk T., El Hajj J., Segal-Bendirdjian E., Hilal G. The long non coding RNA H19 as a biomarker for breast cancer diagnosis in Lebanese women. Sci. Rep. 2020;10:22228. doi: 10.1038/s41598-020-79285-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuel, W., Kirstin, M., Vedran, F., Asija, D., Theresa, G.L., Roberto, A., Filippos, K., David, K., Katja, H., Salah, A., Christopher, B., Karen, H., Anja, R., Ivano, L., Andranik, I., Tommaso, M., Simone, D.G., Jan, P., Samantha, P., Meyer Thomas, F., Alexander, M.M., Daniela, N., Andreas, H., Matthias, S., Altuna, A., Nikolaus, R., Christian, D. and Markus, L., 2021. Transcriptomic profiling of SARS-CoV-2 infected human cell lines identifies HSP90 as target for COVID-19 therapy. iScience, 102151. [DOI] [PMC free article] [PubMed]
- Evans P.C., Rainger G.E., Mason J.C., Guzik T.J., Osto E., Stamataki Z., Neil D., Hoefer I.E., Fragiadaki M., Waltenberger J., Weber C., Bochaton-Piallat M.L., Back M. Endothelial dysfunction in COVID-19: a position paper of the ESC Working Group for Atherosclerosis and Vascular Biology, and the ESC Council of Basic Cardiovascular Science. Cardiovasc. Res. 2020;116:2177–2184. doi: 10.1093/cvr/cvaa230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbri E., Borgatti M., Montagner G., Bianchi N., Finotti A., Lampronti I., Bezzerri V., Dechecchi M.C., Cabrini G., Gambari R. Expression of microRNA-93 and Interleukin-8 during Pseudomonas aeruginosa-mediated induction of proinflammatory responses. Am. J. Respir. Cell Mol. Biol. 2014;50:1144–1155. doi: 10.1165/rcmb.2013-0160OC. [DOI] [PubMed] [Google Scholar]
- Fatica A., Bozzoni I. Long non-coding RNAs: new players in cell differentiation and development. Nat. Rev. Genet. 2014;15:7–21. doi: 10.1038/nrg3606. [DOI] [PubMed] [Google Scholar]
- Fortes P., Morris K.V. Long noncoding RNAs in viral infections. Virus Res. 2016;212:1–11. doi: 10.1016/j.virusres.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox S.E., Li G., Akmatbekov A., Harbert J.L., Lameira F.S., Brown J.Q., Vander Heide R.S. Unexpected Features of Cardiac Pathology in COVID-19 Infection. Circulation. 2020;142:1123–1125. doi: 10.1161/CIRCULATIONAHA.120.049465. [DOI] [PubMed] [Google Scholar]
- Friedman R.C., Farh K.K., Burge C.B., Bartel D.P. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X., Liu P., Dimopoulos G., Zhu J. Dynamic miRNA-mRNA interactions coordinate gene expression in adult Anopheles gambiae. PLoS Genet. 2020;16:e1008765. doi: 10.1371/journal.pgen.1008765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J., Peng L., Tao T., Chen Y., Li Z., Li J. Regulatory roles of the miR-200 family in neurodegenerative diseases. Biomed. Pharmacother. 2019;119 doi: 10.1016/j.biopha.2019.109409. [DOI] [PubMed] [Google Scholar]
- Gao Y.M., Xu G., Wang B., Liu B.C. Cytokine storm syndrome in coronavirus disease 2019: A narrative review. J. Intern. Med. 2020 doi: 10.1111/joim.13144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparello J., Finotti A., Gambari R. Tackling the COVID-19 “cytokine storm” with microRNA mimics directly targeting the 3'UTR of pro-inflammatory mRNAs. Med. Hypotheses. 2021;146 doi: 10.1016/j.mehy.2020.110415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaytán-Pacheco N., Ibáñez-Salazar A., Herrera-Van Oostdam A.S., Oropeza-Valdez J.J., Magaña-Aquino M., Adrián López J., Monárrez-Espino J., López-Hernández Y. miR-146a, miR-221, and miR-155 are Involved in Inflammatory Immune Response in Severe COVID-19 Patients. Diagnostics (Basel) 2022:13. doi: 10.3390/diagnostics13010133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebert L.F.R., MacRae I.J. Regulation of microRNA function in animals. Nat. Rev. Mol. Cell Biol. 2019;20:21–37. doi: 10.1038/s41580-018-0045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh Z., Mallick B., Chakrabarti J. Cellular versus viral microRNAs in host-virus interaction. Nucleic Acids Res. 2009;37:1035–1048. doi: 10.1093/nar/gkn1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girardi E., Lopez P., Pfeffer S. On the Importance of Host MicroRNAs During Viral Infection. Front. Genet. 2018;9:439. doi: 10.3389/fgene.2018.00439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glowacka I., Bertram S., Muller M.A., Allen P., Soilleux E., Pfefferle S., Steffen I., Tsegaye T.S., He Y., Gnirss K., Niemeyer D., Schneider H., Drosten C., Pohlmann S. Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response. J. Virol. 2011;85:4122–4134. doi: 10.1128/JVI.02232-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guay C., Regazzi R. Circulating microRNAs as novel biomarkers for diabetes mellitus. Nat. Rev. Endocrinol. 2013;9:513–521. doi: 10.1038/nrendo.2013.86. [DOI] [PubMed] [Google Scholar]
- Guo J., Huang Z., Lin L., Lv J. Coronavirus Disease 2019 (COVID-19) and Cardiovascular Disease: A Viewpoint on the Potential Influence of Angiotensin-Converting Enzyme Inhibitors/Angiotensin Receptor Blockers on Onset and Severity of Severe Acute Respiratory Syndrome Coronavirus 2 Infection. J. Am. Heart Assoc. 2020;9:e016219. doi: 10.1161/JAHA.120.016219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutbrod M.J., Martienssen R.A. Conserved chromosomal functions of RNA interference. Nat. Rev. Genet. 2020;21:311–331. doi: 10.1038/s41576-019-0203-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guterres A., de Azeredo Lima C.H., Miranda R.L., Gadelha M.R. What is the potential function of microRNAs as biomarkers and therapeutic targets in COVID-19? Infect. Genet. Evol. 2020;85 doi: 10.1016/j.meegid.2020.104417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutschner T., Diederichs S. The hallmarks of cancer: a long non-coding RNA point of view. RNA Biol. 2012;9:703–719. doi: 10.4161/rna.20481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttman M., Amit I., Garber M., French C., Lin M.F., Feldser D., Huarte M., Zuk O., Carey B.W., Cassady J.P., Cabili M.N., Jaenisch R., Mikkelsen T.S., Jacks T., Hacohen N., Bernstein B.E., Kellis M., Regev A., Rinn J.L., Lander E.S. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature. 2009;458:223–227. doi: 10.1038/nature07672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haasnoot J., Berkhout B. RNAi and cellular miRNAs in infections by mammalian viruses. Methods Mol. Biol. 2011;721:23–41. doi: 10.1007/978-1-61779-037-9_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han P., Bartold P.M., Salomon C., Ivanovski S. Salivary Small Extracellular Vesicles Associated miRNAs in Periodontal Status-A Pilot Study. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21082809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Y., Yan Z., Zhang A., Hu S., Wang N., Luo X.G., Ma W., Zhang T.C., He H. IL-6/STAT3 mediates the HPV18 E6/E7 stimulated upregulation of MALAT1 gene in cervical cancer HeLa cells. Virus Res. 2020;281 doi: 10.1016/j.virusres.2020.197907. [DOI] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Muller M.A., Drosten C., Pohlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181(271–280):e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong L., Sharp T., Khorsand B., Fischer C., Eliason S., Salem A., Akkouch A., Brogden K., Amendt B.A. MicroRNA-200c Represses IL-6, IL-8, and CCL-5 Expression and Enhances Osteogenic Differentiation. PLoS One. 2016;11:e0160915. doi: 10.1371/journal.pone.0160915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou J., Wang P., Lin L., Liu X., Ma F., An H., Wang Z., Cao X. MicroRNA-146a feedback inhibits RIG-I-dependent Type I IFN production in macrophages by targeting TRAF6, IRAK1, and IRAK2. J. Immunol. 2009;183:2150–2158. doi: 10.4049/jimmunol.0900707. [DOI] [PubMed] [Google Scholar]
- Hsu S.H., Wang B., Kota J., Yu J., Costinean S., Kutay H., Yu L., Bai S., La Perle K., Chivukula R.R., Mao H., Wei M., Clark K.R., Mendell J.R., Caligiuri M.A., Jacob S.T., Mendell J.T., Ghoshal K. Essential metabolic, anti-inflammatory, and anti-tumorigenic functions of miR-122 in liver. J. Clin. Invest. 2012;122:2871–2883. doi: 10.1172/JCI63539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H.L., Nie Z.Q., Lu Y., Yang X., Song C., Chen H., Zhu S., Chen B.B., Huang J., Geng S., Zhao S. Circulating miR-125b but not miR-125a correlates with acute exacerbations of chronic obstructive pulmonary disease and the expressions of inflammatory cytokines. Medicine (Baltimore) 2017;96:e9059. doi: 10.1097/MD.0000000000009059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J., Wu H., Wang D., Yang Z., Dong J. LncRNA ANRIL promotes NLRP3 inflammasome activation in uric acid nephropathy through miR-122-5p/BRCC3 axis. Biochimie. 2019;157:102–110. doi: 10.1016/j.biochi.2018.10.011. [DOI] [PubMed] [Google Scholar]
- Hu J., Stojanovic J., Yasamineh S., Yasamineh P., Karuppannan S.K., Hussain Dowlath M.J., Serati-Nouri H. The potential use of microRNAs as a therapeutic strategy for SARS-CoV-2 infection. Arch. Virol. 2021;166:2649–2672. doi: 10.1007/s00705-021-05152-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Liu H., Sun X., Ding M., Tao G., Li X. Honeysuckle-derived microRNA2911 directly inhibits varicella-zoster virus replication by targeting IE62 gene. J. Neurovirol. 2019;25:457–463. doi: 10.1007/s13365-019-00741-2. [DOI] [PubMed] [Google Scholar]
- Huang K., Wang C., Vagts C., Raguveer V., Finn P.W., Perkins D.L. Long non-coding RNAs (lncRNAs) NEAT1 and MALAT1 are differentially expressed in severe COVID-19 patients: An integrated single-cell analysis. PLoS One. 2022;17:e0261242. doi: 10.1371/journal.pone.0261242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain S., Pan J., Chen Y., Yang Y., Xu J., Peng Y., Wu Y., Li Z., Zhu Y., Tien P., Guo D. Identification of novel subgenomic RNAs and noncanonical transcription initiation signals of severe acute respiratory syndrome coronavirus. J. Virol. 2005;79:5288–5295. doi: 10.1128/JVI.79.9.5288-5295.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingle H., Kumar S., Raut A.A., Mishra A., Kulkarni D.D., Kameyama T., Takaoka A., Akira S., Kumar H. The microRNA miR-485 targets host and influenza virus transcripts to regulate antiviral immunity and restrict viral replication. Sci. Signal. 2015;8:ra126. doi: 10.1126/scisignal.aab3183. [DOI] [PubMed] [Google Scholar]
- Janssen H.L., Reesink H.W., Lawitz E.J., Zeuzem S., Rodriguez-Torres M., Patel K., van der Meer A.J., Patick A.K., Chen A., Zhou Y., Persson R., King B.D., Kauppinen S., Levin A.A., Hodges M.R. Treatment of HCV infection by targeting microRNA. N. Engl. J. Med. 2013;368:1685–1694. doi: 10.1056/NEJMoa1209026. [DOI] [PubMed] [Google Scholar]
- Jiang C., Li Y., Zhao Z., Lu J., Chen H., Ding N., Wang G., Xu J., Li X. Identifying and functionally characterizing tissue-specific and ubiquitously expressed human lncRNAs. Oncotarget. 2016;7:7120–7133. doi: 10.18632/oncotarget.6859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang N., Meng X., Mi H., Chi Y., Li S., Jin Z., Tian H., He J., Shen W., Tian H., Pan J., Fang S., Jin X., Zhou C., Gong Z. Circulating lncRNA XLOC_009167 serves as a diagnostic biomarker to predict lung cancer. Clin. Chim. Acta. 2018;486:26–33. doi: 10.1016/j.cca.2018.07.026. [DOI] [PubMed] [Google Scholar]
- Jopling C.L., Yi M., Lancaster A.M., Lemon S.M., Sarnow P. Modulation of hepatitis C virus RNA abundance by a liver-specific MicroRNA. Science. 2005;309:1577–1581. doi: 10.1126/science.1113329. [DOI] [PubMed] [Google Scholar]
- Ju Y., Bai H., Ren L., Zhang L. The Role of Exosome and the ESCRT Pathway on Enveloped Virus Infection. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22169060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalarikkal S.P., Sundaram G.M. Edible plant-derived exosomal microRNAs: Exploiting a cross-kingdom regulatory mechanism for targeting SARS-CoV-2. Toxicol. Appl. Pharmacol. 2021;414 doi: 10.1016/j.taap.2021.115425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi E., Azari H., Yari M., Tahmasebi A., Hassani Azad M., Mousavi P. Interplay between SARS-CoV-2-derived miRNAs, immune system, vitamin D pathway and respiratory system. J. Cell Mol. Med. 2021;25:7825–7839. doi: 10.1111/jcmm.16694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M., Castro N.E., Natarajan R. MicroRNAs: potential mediators and biomarkers of diabetic complications. Free Radic. Biol. Med. 2013;64:85–94. doi: 10.1016/j.freeradbiomed.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur T., Kapila S., Kapila R., Kumar S., Upadhyay D., Kaur M., Sharma C. Tmprss2 specific miRNAs as promising regulators for SARS-CoV-2 entry checkpoint. Virus Res. 2021;198275 doi: 10.1016/j.virusres.2020.198275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp V., Laconi A., Cocciolo G., Berends A.J., Breit T.M., Verheije M.H. miRNA repertoire and host immune factor regulation upon avian coronavirus infection in eggs. Arch. Virol. 2020;165:835–843. doi: 10.1007/s00705-020-04527-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M.A., Sany M.R.U., Islam M.S., Islam A. Epigenetic Regulator miRNA Pattern Differences Among SARS-CoV, SARS-CoV-2, and SARS-CoV-2 World-Wide Isolates Delineated the Mystery Behind the Epic Pathogenicity and Distinct Clinical Characteristics of Pandemic COVID-19. Front. Genet. 2020;11:765. doi: 10.3389/fgene.2020.00765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W.R., Park E.G., Kang K.W., Lee S.M., Kim B., Kim H.S. Expression Analyses of MicroRNAs in Hamster Lung Tissues Infected by SARS-CoV-2. Mol. Cells. 2020;43:953–963. doi: 10.14348/molcells.2020.0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak J., Jonak K., Maciejewski R. The function of miR-200 family in oxidative stress response evoked in cancer chemotherapy and radiotherapy. Biomed. Pharmacother. 2020;125 doi: 10.1016/j.biopha.2020.110037. [DOI] [PubMed] [Google Scholar]
- Lahiri D., Mondal R., Deb S., Bandyopadhyay D., Shome G., Sarkar S., Biswas S.C. Neuroinvasive potential of a primary respiratory pathogen SARS- CoV2: Summarizing the evidences. Diabetes Metab. Syndr. 2020;14:1053–1060. doi: 10.1016/j.dsx.2020.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C.C., Shih T.P., Ko W.C., Tang H.J., Hsueh P.R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int. J. Antimicrob. Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai F.W., Stephenson K.B., Mahony J., Lichty B.D. Human coronavirus OC43 nucleocapsid protein binds microRNA 9 and potentiates NF-kappaB activation. J. Virol. 2014;88:54–65. doi: 10.1128/JVI.02678-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert D.W., Lambert L.A., Clarke N.E., Hooper N.M., Porter K.E., Turner A.J. Angiotensin-converting enzyme 2 is subject to post-transcriptional regulation by miR-421. Clin. Sci. (Lond.) 2014;127:243–249. doi: 10.1042/CS20130420. [DOI] [PubMed] [Google Scholar]
- Lanford R.E., Hildebrandt-Eriksen E.S., Petri A., Persson R., Lindow M., Munk M.E., Kauppinen S., Orum H. Therapeutic silencing of microRNA-122 in primates with chronic hepatitis C virus infection. Science. 2010;327:198–201. doi: 10.1126/science.1178178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Hu X., Li L., Li J.H. Differential microRNA expression in the peripheral blood from human patients with COVID-19. J. Clin. Lab. Anal. 2020;34:e23590. doi: 10.1002/jcla.23590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Huang Y., Sun M., Ji H., Dou H., Hu J., Yan Y., Wang X., Chen L. Honeysuckle-encoded microRNA2911 inhibits Enterovirus 71 replication via targeting VP1 gene. Antiviral Res. 2018;152:117–123. doi: 10.1016/j.antiviral.2018.02.015. [DOI] [PubMed] [Google Scholar]
- Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., Somasundaran M., Sullivan J.L., Luzuriaga K., Greenough T.C., Choe H., Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., He C., Wu J., Yang D., Yi W. Epstein barr virus encodes miRNAs to assist host immune escape. J. Cancer. 2020;11:2091–2100. doi: 10.7150/jca.42498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Yang C., Li Y., Chen A., Li L., You Z. LncRNA GAS5 suppresses ovarian cancer by inducing inflammasome formation. Biosci. Rep. 2018;38 doi: 10.1042/BSR20171150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Z., Ren C. Emodin attenuates apoptosis and inflammation induced by LPS through up-regulating lncRNA TUG1 in murine chondrogenic ATDC5 cells. Biomed. Pharmacother. 2018;103:897–902. doi: 10.1016/j.biopha.2018.04.085. [DOI] [PubMed] [Google Scholar]
- Libby P., Luscher T. COVID-19 is, in the end, an endothelial disease. Eur. Heart J. 2020;41:3038–3044. doi: 10.1093/eurheartj/ehaa623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J., Wang Z., Wang J., Yang Q. Microarray analysis of infectious bronchitis virus infection of chicken primary dendritic cells. BMC Genomics. 2019;20:557. doi: 10.1186/s12864-019-5940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Ding C. Roles of LncRNAs in Viral Infections. Front. Cell. Infect. Microbiol. 2017;7:205. doi: 10.3389/fcimb.2017.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Du J., Yu X., Xu J., Huang F., Li X., Zhang C., Li X., Chang J., Shang D., Zhao Y., Tian M., Lu H., Xu J., Li C., Zhu H., Jin N., Jiang C. miRNA-200c-3p is crucial in acute respiratory distress syndrome. Cell Discov. 2017;3:17021. doi: 10.1038/celldisc.2017.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Hu W., Li L.L., Wang Y.X., Zhou Q., Zhang F., Song-Yang Y.Y., Zhu W., Sun C.C., Li D.J. Roles of miR-200 family members in lung cancer: more than tumor suppressors. Future Oncol. 2018;14:2875–2886. doi: 10.2217/fon-2018-0155. [DOI] [PubMed] [Google Scholar]
- Liu D., Tan Q., Zhu J., Zhang Y., Xue Y., Song Y., Liu Y., Wang Q., Lai L. MicroRNA-33/33* inhibit the activation of MAVS through AMPK in antiviral innate immunity. Cell. Mol. Immunol. 2021;18:1450–1462. doi: 10.1038/s41423-019-0326-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Wang F., Du L., Li J., Yu T., Jin Y., Yan Y., Zhou J., Gu J. Comprehensive Genomic Characterization Analysis of lncRNAs in Cells With Porcine Delta Coronavirus Infection. Front. Microbiol. 2019;10:3036. doi: 10.3389/fmicb.2019.03036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Wang X. Prediction of functional microRNA targets by integrative modeling of microRNA binding and target expression data. Genome Biol. 2019;20:18. doi: 10.1186/s13059-019-1629-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge R., Ferreira Barbosa J.A., Lombard-Vadnais F., Gilmore J.C., Deshiere A., Gosselin A., Wiche Salinas T.R., Bego M.G., Power C., Routy J.P., Ancuta P., Tremblay M.J., Cohen E.A. Host MicroRNAs-221 and -222 Inhibit HIV-1 Entry in Macrophages by Targeting the CD4 Viral Receptor. Cell Rep. 2017;21:141–153. doi: 10.1016/j.celrep.2017.09.030. [DOI] [PubMed] [Google Scholar]
- Lodhi N., Singh R., Rajput S.P., Saquib Q. SARS-CoV-2: Understanding the Transcriptional Regulation of ACE2 and TMPRSS2 and the Role of Single Nucleotide Polymorphism (SNP) at Codon 72 of p53 in the Innate Immune Response against Virus Infection. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22168660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu D., Chatterjee S., Xiao K., Riedel I., Wang Y., Foo R., Bar C., Thum T. MicroRNAs targeting the SARS-CoV-2 entry receptor ACE2 in cardiomyocytes. J. Mol. Cell. Cardiol. 2020;148:46–49. doi: 10.1016/j.yjmcc.2020.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N., Bi Y., Ma X., Zhan F., Wang L., Hu T., Zhou H., Hu Z., Zhou W., Zhao L., Chen J., Meng Y., Wang J., Lin Y., Yuan J., Xie Z., Ma J., Liu W.J., Wang D., Xu W., Holmes E.C., Gao G.F., Wu G., Chen W., Shi W., Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X., Wang X., Yang C., Wang Z., Han B., Wu L., Zhuang L. DANCR Acts as a Diagnostic Biomarker and Promotes Tumor Growth and Metastasis in Hepatocellular Carcinoma. Anticancer Res. 2016;36:6389–6398. doi: 10.21873/anticanres.11236. [DOI] [PubMed] [Google Scholar]
- Ma Y., Wang C., Xue M., Fu F., Zhang X., Li L., Yin L., Xu W., Feng L., Liu P. The Coronavirus Transmissible Gastroenteritis Virus Evades the Type I Interferon Response through IRE1alpha-Mediated Manipulation of the MicroRNA miR-30a-5p/SOCS1/3 Axis. J. Virol. 2018;92 doi: 10.1128/JVI.00728-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X., Zhao X., Zhang Z., Guo J., Guan L., Li J., Mi M., Huang Y., Tong D. Differentially expressed non-coding RNAs induced by transmissible gastroenteritis virus potentially regulate inflammation and NF-kappaB pathway in porcine intestinal epithelial cell line. BMC Genomics. 2018;19:747. doi: 10.1186/s12864-018-5128-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X., Zhao X., Wang K., Tang X., Guo J., Mi M., Qi Y., Chang L., Huang Y., Tong D. Identification and analysis of long non-coding RNAs that are involved in inflammatory process in response to transmissible gastroenteritis virus infection. BMC Genomics. 2019;20:806. doi: 10.1186/s12864-019-6156-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallick B., Ghosh Z., Chakrabarti J. MicroRNome analysis unravels the molecular basis of SARS infection in bronchoalveolar stem cells. PLoS One. 2009;4:e7837. doi: 10.1371/journal.pone.0007837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matarese A., Gambardella J., Sardu C., Santulli G. miR-98 Regulates TMPRSS2 Expression in Human Endothelial Cells: Key Implications for COVID-19. Biomedicines. 2020;8 doi: 10.3390/biomedicines8110462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathy N.W., Chen X.M. Long non-coding RNAs (lncRNAs) and their transcriptional control of inflammatory responses. J. Biol. Chem. 2017;292:12375–12382. doi: 10.1074/jbc.R116.760884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaskill J.L., Ressel S., Alber A., Redford J., Power U.F., Schwarze J., Dutia B.M., Buck A.H. Broad-Spectrum Inhibition of Respiratory Virus Infection by MicroRNA Mimics Targeting p38 MAPK Signaling. Mol. Ther. Nucleic Acids. 2017;7:256–266. doi: 10.1016/j.omtn.2017.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald, J.T., Enguita, F.J., Taylor, D., Griffin, R.J., Priebe, W., Emmett, M.R., Sajadi, M.M., Harris, A.D., Clement, J., Dybas, J.M., Aykin-Burns, N., Guarnieri, J.W., Singh, L.N., Grabham, P., Baylin, S.B., Yousey, A., Pearson, A.N., Corry, P.M., Saravia-Butler, A., Aunins, T.R., Sharma, S., Nagpal, P., Meydan, C., Foox, J., Mozsary, C., Cerqueira, B., Zaksas, V., Singh, U., Wurtele, E.S., Costes, S.V., Davanzo, G.G., Galeano, D., Paccanaro, A., Meinig, S.L., Hagan, R.S., Bowman, N.M., Consortium, U.C.-P., Wolfgang, M.C., Altinok, S., Sapoval, N., Treangen, T.J., Moraes-Vieira, P.M., Vanderburg, C., Wallace, D.C., Schisler, J.C., Mason, C.E., Chatterjee, A., Meller, R. and Beheshti, A., 2021. Role of miR-2392 in driving SARS-CoV-2 infection. Cell Rep 37, 109839. [DOI] [PMC free article] [PubMed]
- McIntosh K., Becker W.B., Chanock R.M. Growth in suckling-mouse brain of “IBV-like” viruses from patients with upper respiratory tract disease. PNAS. 1967;58:2268–2273. doi: 10.1073/pnas.58.6.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta A., Baltimore D. MicroRNAs as regulatory elements in immune system logic. Nat. Rev. Immunol. 2016;16:279–294. doi: 10.1038/nri.2016.40. [DOI] [PubMed] [Google Scholar]
- Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J., Collaboration H.A.S., U.k., COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meydan C., Madrer N., Soreq H. The Neat Dance of COVID-19: NEAT1, DANCR, and Co-Modulated Cholinergic RNAs Link to Inflammation. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.590870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi S., Zhang J., Zhang W., Huang R.S. Circulating microRNAs as biomarkers for inflammatory diseases. Microrna. 2013;2:63–71. doi: 10.2174/2211536611302010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel C.J., Mayer C., Poch O., Thompson J.D. Characterization of accessory genes in coronavirus genomes. Virol. J. 2020;17:131. doi: 10.1186/s12985-020-01402-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra, R., Bhattacharya, S., Rawat, B.S., Kumar, A., Kumar, A., Niraj, K., Chande, A., Gandhi, P., Khetan, D., Aggarwal, A., Sato, S., Tailor, P., Takaoka, A. and Kumar, H., 2020. MicroRNA-30e-5p has an Integrated Role in the Regulation of the Innate Immune Response during Virus Infection and Systemic Lupus Erythematosus. iScience 23, 101322. [DOI] [PMC free article] [PubMed]
- Mukhopadhyay, D. and Mussa, B.M., 2020. Identification of Novel Hypothalamic MicroRNAs as Promising Therapeutics for SARS-CoV-2 by Regulating ACE2 and TMPRSS2 Expression: An In Silico Analysis. Brain Sci 10. [DOI] [PMC free article] [PubMed]
- Neeb Z.T., Ritter A.J., Chauhan L.V., Katzman S., Lipkin W.I., Mishra N., Sanford J.R. A potential role for SARS-CoV-2 small viral RNAs in targeting host microRNAs and modulating gene expression. Sci. Rep. 2022;12:21694. doi: 10.1038/s41598-022-26135-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nersisyan S., Shkurnikov M., Turchinovich A., Knyazev E., Tonevitsky A. Integrative analysis of miRNA and mRNA sequencing data reveals potential regulatory mechanisms of ACE2 and TMPRSS2. PLoS One. 2020;15:e0235987. doi: 10.1371/journal.pone.0235987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T.H., Liu X., Su Z.Z., Hsu A.C., Foster P.S., Yang M. Potential Role of MicroRNAs in the Regulation of Antiviral Responses to Influenza Infection. Front. Immunol. 2018;9:1541. doi: 10.3389/fimmu.2018.01541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomburg J., Meyerson M., DeCaprio J.A. Pervasive generation of non-canonical subgenomic RNAs by SARS-CoV-2. Genome Med. 2020;12:108. doi: 10.1186/s13073-020-00802-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien J., Hayder H., Zayed Y., Peng C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front Endocrinol (Lausanne) 2018;9:402. doi: 10.3389/fendo.2018.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oglesby I.K., Vencken S.F., Agrawal R., Gaughan K., Molloy K., Higgins G., McNally P., McElvaney N.G., Mall M.A., Greene C.M. miR-17 overexpression in cystic fibrosis airway epithelial cells decreases interleukin-8 production. Eur. Respir. J. 2015;46:1350–1360. doi: 10.1183/09031936.00163414. [DOI] [PubMed] [Google Scholar]
- Panda M., Kalita E., Singh S., Kumar K., Rao A., Prajapati V.K. MiRNA-SARS-CoV-2 dialogue and prospective anti-COVID-19 therapies. Life Sci. 2022;305 doi: 10.1016/j.lfs.2022.120761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paniri A., Akhavan-Niaki H. Emerging role of IL-6 and NLRP3 inflammasome as potential therapeutic targets to combat COVID-19: Role of lncRNAs in cytokine storm modulation. Life Sci. 2020;257 doi: 10.1016/j.lfs.2020.118114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick D.M., Petric M., Skowronski D.M., Guasparini R., Booth T.F., Krajden M., McGeer P., Bastien N., Gustafson L., Dubord J., Macdonald D., David S.T., Srour L.F., Parker R., Andonov A., Isaac-Renton J., Loewen N., McNabb G., McNabb A., Goh S.H., Henwick S., Astell C., Guo J.P., Drebot M., Tellier R., Plummer F., Brunham R.C. An Outbreak of Human Coronavirus OC43 Infection and Serological Cross-reactivity with SARS Coronavirus. Can. J. Infect. Dis. Med. Microbiol. 2006;17:330–336. doi: 10.1155/2006/152612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S., Bravo Vázquez L.A., Reyes-Pérez P.R., Estrada-Meza C., Aponte Alburquerque R.A., Pathak S., Banerjee A., Bandyopadhyay A., Chakraborty S., Srivastava A. The role of microRNAs in solving COVID-19 puzzle from infection to therapeutics: A mini-review. Virus Res. 2022;308 doi: 10.1016/j.virusres.2021.198631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlica, P., Yario, T.A., White, S., Wang, J., Moss, W.N., Hui, P., Vinetz, J.M. and Steitz, J.A., 2021. SARS-CoV-2 expresses a microRNA-like small RNA able to selectively repress host genes. Proceedings of the National Academy of Sciences 118, e2116668118. [DOI] [PMC free article] [PubMed]
- Pearson M.J., Jones S.W. Review: Long Noncoding RNAs in the Regulation of Inflammatory Pathways in Rheumatoid Arthritis and Osteoarthritis. Arthritis Rheumatol. 2016;68:2575–2583. doi: 10.1002/art.39759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, X., Gralinski, L., Armour, C.D., Ferris, M.T., Thomas, M.J., Proll, S., Bradel-Tretheway, B.G., Korth, M.J., Castle, J.C., Biery, M.C., Bouzek, H.K., Haynor, D.R., Frieman, M.B., Heise, M., Raymond, C.K., Baric, R.S. and Katze, M.G., 2010. Unique signatures of long noncoding RNA expression in response to virus infection and altered innate immune signaling. mBio 1. [DOI] [PMC free article] [PubMed]
- Peng, X., Gralinski, L., Ferris, M.T., Frieman, M.B., Thomas, M.J., Proll, S., Korth, M.J., Tisoncik, J.R., Heise, M., Luo, S., Schroth, G.P., Tumpey, T.M., Li, C., Kawaoka, Y., Baric, R.S. and Katze, M.G., 2011. Integrative deep sequencing of the mouse lung transcriptome reveals differential expression of diverse classes of small RNAs in response to respiratory virus infection. mBio 2. [DOI] [PMC free article] [PubMed]
- Pennisi M., Lanza G., Falzone L., Fisicaro F., Ferri R., Bella R. SARS-CoV-2 and the Nervous System: From Clinical Features to Molecular Mechanisms. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21155475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman S. Another Decade, Another Coronavirus. N. Engl. J. Med. 2020;382:760–762. doi: 10.1056/NEJMe2001126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce, J.B., Simion, V., Icli, B., Perez-Cremades, D., Cheng, H.S. and Feinberg, M.W., 2020. Computational Analysis of Targeting SARS-CoV-2, Viral Entry Proteins ACE2 and TMPRSS2, and Interferon Genes by Host MicroRNAs. Genes (Basel) 11. [DOI] [PMC free article] [PubMed]
- Pinilla L., Benitez I.D., Gonzalez J., Torres G., Barbe F., de Gonzalo-Calvo D. Peripheral blood microRNAs and the COVID-19 patient: methodological considerations, technical challenges and practice points. RNA Biol. 2021 doi: 10.1080/15476286.2021.1885188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pottoo F.H., Javed M.N., Rahman J.U., Abu-Izneid T., Khan F.A. Targeted delivery of miRNA based therapeuticals in the clinical management of Glioblastoma Multiforme. Semin. Cancer Biol. 2020 doi: 10.1016/j.semcancer.2020.04.001. [DOI] [PubMed] [Google Scholar]
- Qiu L., Wang T., Tang Q., Li G., Wu P., Chen K. Long Non-coding RNAs: Regulators of Viral Infection and the Interferon Antiviral Response. Front. Microbiol. 2018;9:1621. doi: 10.3389/fmicb.2018.01621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan J., Pan X., Zhao L., Li Z., Dai K., Yan F., Liu S., Ma H., Lai Y. LncRNA as a diagnostic and prognostic biomarker in bladder cancer: a systematic review and meta-analysis. Onco Targets Ther. 2018;11:6415–6424. doi: 10.2147/OTT.S167853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radovic N., Nikolic Jakoba N., Petrovic N., Milosavljevic A., Brkovic B., Roganovic J. MicroRNA-146a and microRNA-155 as novel crevicular fluid biomarkers for periodontitis in non-diabetic and type 2 diabetic patients. J. Clin. Periodontol. 2018;45:663–671. doi: 10.1111/jcpe.12888. [DOI] [PubMed] [Google Scholar]
- Ramezani A., Devaney J.M., Cohen S., Wing M.R., Scott R., Knoblach S., Singhal R., Howard L., Kopp J.B., Raj D.S. Circulating and urinary microRNA profile in focal segmental glomerulosclerosis: a pilot study. Eur. J. Clin. Invest. 2015;45:394–404. doi: 10.1111/eci.12420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rarani, F.Z., Rashidi, B., Jafari Najaf Abadi, M.H., Hamblin, M.R., Reza Hashemian, S.M. and Mirzaei, H., 2022. Cytokines and microRNAs in SARS-CoV-2: What do we know? Mol Ther Nucleic Acids 29, 219-242. [DOI] [PMC free article] [PubMed]
- Reich H.N., Oudit G.Y., Penninger J.M., Scholey J.W., Herzenberg A.M. Decreased glomerular and tubular expression of ACE2 in patients with type 2 diabetes and kidney disease. Kidney Int. 2008;74:1610–1616. doi: 10.1038/ki.2008.497. [DOI] [PubMed] [Google Scholar]
- Rodrigues, A.C., Adamoski, D., Genelhould, G., Zhen, F., Yamaguto, G.E., Souza, P.S.A., Nogueira, M.B., Raboni, S.M., Bonatto, A.C., Gradia, D.F. and de Oliveira, J.C., 2021. NEAT1 and MALAT1 are highly expressed in saliva and nasopharyngeal swab sample of COVID-19 patients. Mol Oral Microbiol. [DOI] [PMC free article] [PubMed]
- Roganovic J.R. microRNA-146a and -155, upregulated by periodontitis and type 2 diabetes in oral fluids, are predicted to regulate SARS-CoV-2 oral receptors genes. J. Periodontol. 2020 doi: 10.1002/JPER.20-0623. [DOI] [PubMed] [Google Scholar]
- Rossato M., Affandi A.J., Thordardottir S., Wichers C.G.K., Cossu M., Broen J.C.A., Moret F.M., Bossini-Castillo L., Chouri E., van Bon L., Wolters F., Marut W., van der Kroef M., Silva-Cardoso S., Bekker C.P.J., Dolstra H., van Laar J.M., Martin J., van Roon J.A.G., Reedquist K.A., Beretta L., Radstake T. Association of MicroRNA-618 Expression With Altered Frequency and Activation of Plasmacytoid Dendritic Cells in Patients With Systemic Sclerosis. Arthritis Rheumatol. 2017;69:1891–1902. doi: 10.1002/art.40163. [DOI] [PubMed] [Google Scholar]
- Saba R., Sorensen D.L., Booth S.A. MicroRNA-146a: A Dominant, Negative Regulator of the Innate Immune Response. Front. Immunol. 2014;5:578. doi: 10.3389/fimmu.2014.00578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saberinia A., Alinezhad A., Jafari F., Soltany S., Akhavan Sigari R. Oncogenic miRNAs and target therapies in colorectal cancer. Clin. Chim. Acta. 2020;508:77–91. doi: 10.1016/j.cca.2020.05.012. [DOI] [PubMed] [Google Scholar]
- Sanders C.J., Doherty P.C., Thomas P.G. Respiratory epithelial cells in innate immunity to influenza virus infection. Cell Tissue Res. 2011;343:13–21. doi: 10.1007/s00441-010-1043-z. [DOI] [PubMed] [Google Scholar]
- Sardu, C., Gambardella, J., Morelli, M.B., Wang, X., Marfella, R. and Santulli, G., 2020. Hypertension, Thrombosis, Kidney Failure, and Diabetes: Is COVID-19 an Endothelial Disease? A Comprehensive Evaluation of Clinical and Basic Evidence. J Clin Med 9. [DOI] [PMC free article] [PubMed]
- Shah P., Cho S.K., Thulstrup P.W., Bjerrum M.J., Lee P.H., Kang J.H., Bhang Y.J., Yang S.W. MicroRNA Biomarkers in Neurodegenerative Diseases and Emerging Nano-Sensors Technology. J Mov Disord. 2017;10:18–28. doi: 10.14802/jmd.16037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi T., Gao G., Cao Y. Long Noncoding RNAs as Novel Biomarkers Have a Promising Future in Cancer Diagnostics. Dis. Markers. 2016;2016:9085195. doi: 10.1155/2016/9085195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulla A., Heald-Sargent T., Subramanya G., Zhao J., Perlman S., Gallagher T. A transmembrane serine protease is linked to the severe acute respiratory syndrome coronavirus receptor and activates virus entry. J. Virol. 2011;85:873–882. doi: 10.1128/JVI.02062-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si Z., Zhou S., Shen Z., Luan F., Yan J. lncRNA HAND2-AS1 is downregulated in osteoarthritis and regulates IL-6 expression in chondrocytes. J. Orthop. Surg. Res. 2021;16:68. doi: 10.1186/s13018-021-02216-9. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Hosseini Rad Sm, A. and McLellan, A.D., 2020. Implications of SARS-CoV-2 Mutations for Genomic RNA Structure and Host microRNA Targeting. Int J Mol Sci 21. [DOI] [PMC free article] [PubMed]
- Smith J.L., Jeng S., McWeeney S.K., Hirsch A.J. A MicroRNA Screen Identifies the Wnt Signaling Pathway as a Regulator of the Interferon Response during Flavivirus Infection. J. Virol. 2017;91 doi: 10.1128/JVI.02388-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L., Liu H., Gao S., Jiang W., Huang W. Cellular microRNAs inhibit replication of the H1N1 influenza A virus in infected cells. J. Virol. 2010;84:8849–8860. doi: 10.1128/JVI.00456-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonkoly E., Pivarcsi A. microRNAs in inflammation. Int. Rev. Immunol. 2009;28:535–561. doi: 10.3109/08830180903208303. [DOI] [PubMed] [Google Scholar]
- Stahl K., Brasen J.H., Hoeper M.M., David S. Direct evidence of SARS-CoV-2 in gut endothelium. Intensive Care Med. 2020;46:2081–2082. doi: 10.1007/s00134-020-06237-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statello L., Guo C.-J., Chen L.-L., Huarte M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 2021;22:96–118. doi: 10.1038/s41580-020-00315-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H., Gao Y., Li Z., Miao Y., Huang Z., Liu X., Xie L., Li H., Wen W., Zheng Y., Su W. The noncoding and coding transcriptional landscape of the peripheral immune response in patients with COVID-19. Clin. Transl. Med. 2020;10:e200. doi: 10.1002/ctm2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X., Lan T., Wu R., Zhou Z., Chen Y., Sun Y., Zheng Y., Ma J. Analysis of long non-coding RNAs in neonatal piglets at different stages of porcine deltacoronavirus infection. BMC Vet. Res. 2019;15:111. doi: 10.1186/s12917-019-1862-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y., Liu J., Zhang D., Xu Z., Ji J., Wen C. Cytokine Storm in COVID-19: The Current Evidence and Treatment Strategies. Front. Immunol. 2020;11:1708. doi: 10.3389/fimmu.2020.01708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay M.Z., Poh C.M., Renia L., MacAry P.A., Ng L.F.P. The trinity of COVID-19: immunity, inflammation and intervention. Nat. Rev. Immunol. 2020;20:363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tayel S.I., El-Masry E.A., Abdelaal G.A., Shehab-Eldeen S., Essa A., Muharram N.M. Interplay of LncRNAs NEAT1 and TUG1 in Incidence of Cytokine Storm in Appraisal of COVID-19 Infection. Int. J. Biol. Sci. 2022;18:4901–4913. doi: 10.7150/ijbs.72318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teodori L., Sestili P., Madiai V., Coppari S., Fraternale D., Rocchi M.B.L., Ramakrishna S., Albertini M.C. MicroRNAs Bioinformatics Analyses Identifying HDAC Pathway as a Putative Target for Existing Anti-COVID-19 Therapeutics. Front. Pharmacol. 2020;11 doi: 10.3389/fphar.2020.582003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thai T.H., Calado D.P., Casola S., Ansel K.M., Xiao C., Xue Y., Murphy A., Frendewey D., Valenzuela D., Kutok J.L., Schmidt-Supprian M., Rajewsky N., Yancopoulos G., Rao A., Rajewsky K. Regulation of the germinal center response by microRNA-155. Science. 2007;316:604–608. doi: 10.1126/science.1141229. [DOI] [PubMed] [Google Scholar]
- Tian H., Wu M., Zhou P., Huang C., Ye C., Wang L. The long non-coding RNA MALAT1 is increased in renal ischemia-reperfusion injury and inhibits hypoxia-induced inflammation. Ren. Fail. 2018;40:527–533. doi: 10.1080/0886022X.2018.1487863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triboulet R., Mari B., Lin Y.L., Chable-Bessia C., Bennasser Y., Lebrigand K., Cardinaud B., Maurin T., Barbry P., Baillat V., Reynes J., Corbeau P., Jeang K.T., Benkirane M. Suppression of microRNA-silencing pathway by HIV-1 during virus replication. Science. 2007;315:1579–1582. doi: 10.1126/science.1136319. [DOI] [PubMed] [Google Scholar]
- Trobaugh D.W., Gardner C.L., Sun C., Haddow A.D., Wang E., Chapnik E., Mildner A., Weaver S.C., Ryman K.D., Klimstra W.B. RNA viruses can hijack vertebrate microRNAs to suppress innate immunity. Nature. 2014;506:245–248. doi: 10.1038/nature12869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trobaugh D.W., Klimstra W.B. MicroRNA Regulation of RNA Virus Replication and Pathogenesis. Trends Mol. Med. 2017;23:80–93. doi: 10.1016/j.molmed.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tufan A., Avanoglu Guler A., Matucci-Cerinic M. COVID-19, immune system response, hyperinflammation and repurposing antirheumatic drugs. Turk. J. Med. Sci. 2020;50:620–632. doi: 10.3906/sag-2004-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turjya R.R., Khan M.A., Khademul M.M., Islam A.B. Perversely expressed long noncoding RNAs can alter host response and viral proliferation in SARS-CoV-2 infection. Future Virol. 2020;15:577–593. doi: 10.2217/fvl-2020-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vabret N., Britton G.J., Gruber C., Hegde S., Kim J., Kuksin M., Levantovsky R., Malle L., Moreira A., Park M.D., Pia L., Risson E., Saffern M., Salome B., Esai Selvan M., Spindler M.P., Tan J., van der Heide V., Gregory J.K., Alexandropoulos K., Bhardwaj N., Brown B.D., Greenbaum B., Gumus Z.H., Homann D., Horowitz A., Kamphorst A.O., Curotto de Lafaille M.A., Mehandru S., Merad M., Samstein R.M., Review S.I., P., Immunology of COVID-19: Current State of the Science. Immunity. 2020;52:910–941. doi: 10.1016/j.immuni.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Sand L., Braß P., Gregorius J., Pattberg K., Engler A., Dittmer U., Taube C., Brock S., Berger M.M., Brenner T., Witzke O., Krawczyk A. Upregulation of miRNA-200c during Disease Progression in COVID-19 Patients. J. Clin. Med. 2022;12 doi: 10.3390/jcm12010283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg M.M.J., Krauskopf J., Ramaekers J.G., Kleinjans J.C.S., Prickaerts J., Briede J.J. Circulating microRNAs as potential biomarkers for psychiatric and neurodegenerative disorders. Prog. Neurobiol. 2020;185 doi: 10.1016/j.pneurobio.2019.101732. [DOI] [PubMed] [Google Scholar]
- van der Ree M.H., van der Meer A.J., de Bruijne J., Maan R., van Vliet A., Welzel T.M., Zeuzem S., Lawitz E.J., Rodriguez-Torres M., Kupcova V., Wiercinska-Drapalo A., Hodges M.R., Janssen H.L., Reesink H.W. Long-term safety and efficacy of microRNA-targeted therapy in chronic hepatitis C patients. Antiviral Res. 2014;111:53–59. doi: 10.1016/j.antiviral.2014.08.015. [DOI] [PubMed] [Google Scholar]
- van der Ree M.H., de Vree J.M., Stelma F., Willemse S., van der Valk M., Rietdijk S., Molenkamp R., Schinkel J., van Nuenen A.C., Beuers U., Hadi S., Harbers M., van der Veer E., Liu K., Grundy J., Patick A.K., Pavlicek A., Blem J., Huang M., Grint P., Neben S., Gibson N.W., Kootstra N.A., Reesink H.W. Safety, tolerability, and antiviral effect of RG-101 in patients with chronic hepatitis C: a phase 1B, double-blind, randomised controlled trial. Lancet. 2017;389:709–717. doi: 10.1016/S0140-6736(16)31715-9. [DOI] [PubMed] [Google Scholar]
- Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., Mehra M.R., Schuepbach R.A., Ruschitzka F., Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishnubalaji, R., Shaath, H. and Alajez, N.M., 2020. Protein Coding and Long Noncoding RNA (lncRNA) Transcriptional Landscape in SARS-CoV-2 Infected Bronchial Epithelial Cells Highlight a Role for Interferon and Inflammatory Response. Genes (Basel) 11. [DOI] [PMC free article] [PubMed]
- Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell. 2020;181(281–292):e6. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P. The Opening of Pandora's Box: An Emerging Role of Long Noncoding RNA in Viral Infections. Front. Immunol. 2018;9:3138. doi: 10.3389/fimmu.2018.03138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G., Szeto C.C. Quantification of gene expression in urinary sediment for the study of renal diseases. Nephrology (Carlton) 2007;12:494–499. doi: 10.1111/j.1440-1797.2007.00836.x. [DOI] [PubMed] [Google Scholar]
- Wang C., Yue S., Jiang Y., Mao Y., Zhao Z., Liu X., Zhang X., Pei D., Li Y. LncRNA GAS5 is upregulated in polycystic ovary syndrome and regulates cell apoptosis and the expression of IL-6. J Ovarian Res. 2020;13:145. doi: 10.1186/s13048-020-00748-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L., Li J., Han Z., Chen Z., Zhang Q. Silencing of lncRNA MALAT1 Prevents Inflammatory Injury after Lung Transplant Ischemia-Reperfusion by Downregulation of IL-8 via p300. Mol. Ther. Nucleic Acids. 2019;18:285–297. doi: 10.1016/j.omtn.2019.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widiasta A., Sribudiani Y., Nugrahapraja H., Hilmanto D., Sekarwana N., Rachmadi D. Potential role of ACE2-related microRNAs in COVID-19-associated nephropathy. Noncoding RNA Res. 2020;5:153–166. doi: 10.1016/j.ncrna.2020.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkle M., El-Daly S.M., Fabbri M., Calin G.A. Noncoding RNA therapeutics - challenges and potential solutions. Nat. Rev. Drug Discov. 2021;20:629–651. doi: 10.1038/s41573-021-00219-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Xu X., Chen Z., Duan J., Hashimoto K., Yang L., Liu C., Yang C. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav. Immun. 2020;87:18–22. doi: 10.1016/j.bbi.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Zhao T., Deng R., Xia X., Li B., Wang X. A study of differential circRNA and lncRNA expressions in COVID-19-infected peripheral blood. Sci. Rep. 2021;11:7991. doi: 10.1038/s41598-021-86134-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y., Xiao T., Ou W., Wu Z., Wu J., Tang J., Tian B., Zhou Y., Su M., Wang W. LncRNA SNHG16 as a potential biomarker and therapeutic target in human cancers. Biomark Res. 2020;8:41. doi: 10.1186/s40364-020-00221-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T., Li L.X., Jia Y., Wu Q., Zhu W., Xu Z., Zheng B., Lu X. One microRNA has the potential to target whole viral mRNAs in a given human coronavirus. Front. Microbiol. 2022;13:1035044. doi: 10.3389/fmicb.2022.1035044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan B., Ma H., Jiang S., Shi J., Yang Z., Zhu W., Kong C., Chen L., Yan H., Ma C. microRNA-221 restricts human cytomegalovirus replication via promoting type I IFN production by targeting SOCS1/NF-kappaB pathway. Cell Cycle. 2019;18:3072–3084. doi: 10.1080/15384101.2019.1667706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi H., Peng R., Zhang L.Y., Sun Y., Peng H.M., Liu H.D., Yu L.J., Li A.L., Zhang Y.J., Jiang W.H., Zhang Z. LincRNA-Gm4419 knockdown ameliorates NF-kappaB/NLRP3 inflammasome-mediated inflammation in diabetic nephropathy. Cell Death Dis. 2017;8:e2583. doi: 10.1038/cddis.2016.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi K., Zhang Y., Wang Y., Wang Z., Xie M., Jin Z., Zhao T. Long noncoding RNA and its role in virus infection and pathogenesis. Front Biosci (Landmark Ed) 2019;24:777–789. doi: 10.2741/4750. [DOI] [PubMed] [Google Scholar]
- Ying H., Ebrahimi M., Keivan M., Khoshnam S.E., Salahi S., Farzaneh M. miRNAs; a novel strategy for the treatment of COVID-19. Cell Biol. Int. 2021;45:2045–2053. doi: 10.1002/cbin.11653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S.Y., Dong B., Tang L., Zhou S.H. LncRNA MALAT1 sponges miR-133 to promote NLRP3 inflammasome expression in ischemia-reperfusion injured heart. Int. J. Cardiol. 2018;254:50. doi: 10.1016/j.ijcard.2017.10.071. [DOI] [PubMed] [Google Scholar]
- Yu B., Qi Y., Li R., Shi Q., Satpathy A.T., Chang H.Y. B cell-specific XIST complex enforces X-inactivation and restrains atypical B cells. Cell. 2021 doi: 10.1016/j.cell.2021.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan W., Sun Y., Liu L., Zhou B., Wang S., Gu D. Circulating LncRNAs Serve as Diagnostic Markers for Hepatocellular Carcinoma. Cell. Physiol. Biochem. 2017;44:125–132. doi: 10.1159/000484589. [DOI] [PubMed] [Google Scholar]
- Yuki K., Fujiogi M., Koutsogiannaki S. COVID-19 pathophysiology: A review. Clin. Immunol. 2020;215 doi: 10.1016/j.clim.2020.108427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng F.R., Tang L.J., He Y., Garcia R.C. An update on the role of miRNA-155 in pathogenic microbial infections. Microbes Infect. 2015;17:613–621. doi: 10.1016/j.micinf.2015.05.007. [DOI] [PubMed] [Google Scholar]
- Zhang P., Cao L., Zhou R., Yang X., Wu M. The lncRNA Neat1 promotes activation of inflammasomes in macrophages. Nat. Commun. 2019;10:1495. doi: 10.1038/s41467-019-09482-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Chu M. Targeting of IL-6-Relevant Long Noncoding RNA Profiles in Inflammatory and Tumorous Disease. Inflammation. 2019;42:1139–1146. doi: 10.1007/s10753-019-00995-2. [DOI] [PubMed] [Google Scholar]
- Zhang C., Wang J., Ma X., Wang W., Zhao B., Chen Y., Chen C., Bihl J.C. ACE2-EPC-EXs protect ageing ECs against hypoxia/reoxygenation-induced injury through the miR-18a/Nox2/ROS pathway. J. Cell Mol. Med. 2018;22:1873–1882. doi: 10.1111/jcmm.13471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Bai X., Guan L., Li J., Song X., Ma X., Guo J., Zhang Z., Du Q., Huang Y., Tong D. microRNA-4331 Promotes Transmissible Gastroenteritis Virus (TGEV)-induced Mitochondrial Damage Via Targeting RB1, Upregulating Interleukin-1 Receptor Accessory Protein (IL1RAP), and Activating p38 MAPK Pathway In Vitro. Mol. Cell. Proteomics. 2018;17:190–204. doi: 10.1074/mcp.RA117.000432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q., Xiong Y., Xu J., Chen S., Li P., Huang Y., Wang Y., Chen W.X., Wang B. Host MicroRNA hsa-miR-494-3p Promotes EV71 Replication by Directly Targeting PTEN. Front. Cell. Infect. Microbiol. 2018;8:278. doi: 10.3389/fcimb.2018.00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L., Zhu J., Zhou H., Zhao Z., Zou Z., Liu X., Lin X., Zhang X., Deng X., Wang R., Chen H., Jin M. Identification of cellular microRNA-136 as a dual regulator of RIG-I-mediated innate immunity that antagonizes H5N1 IAV replication in A549 cells. Sci. Rep. 2015;5:14991. doi: 10.1038/srep14991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z., Ke X., Wang M., He S., Li Q., Zheng C., Zhang Z., Liu Y., Wang H. Human microRNA hsa-miR-296-5p suppresses enterovirus 71 replication by targeting the viral genome. J. Virol. 2013;87:5645–5656. doi: 10.1128/JVI.02655-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H., Xu L., Liu Y., Li C., Zhang L., Wang T., Zhao D., Xu X., Zhang Y. MicroRNA-221-5p Inhibits Porcine Epidemic Diarrhea Virus Replication by Targeting Genomic Viral RNA and Activating the NF-kappaB Pathway. Int. J. Mol. Sci. 2018;19 doi: 10.3390/ijms19113381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H., Huang X., Cui H., Luo X., Tang Y., Chen S., Wu L., Shen N. miR-155 and its star-form partner miR-155* cooperatively regulate type I interferon production by human plasmacytoid dendritic cells. Blood. 2010;116:5885–5894. doi: 10.1182/blood-2010-04-280156. [DOI] [PubMed] [Google Scholar]
- Zhou Z., Li X., Liu J., Dong L., Chen Q., Liu J., Kong H., Zhang Q., Qi X., Hou D., Zhang L., Zhang G., Liu Y., Zhang Y., Li J., Wang J., Chen X., Wang H., Zhang J., Chen H., Zen K., Zhang C.Y. Honeysuckle-encoded atypical microRNA2911 directly targets influenza A viruses. Cell Res. 2015;25:39–49. doi: 10.1038/cr.2014.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler C.G.K., Allon S.J., Nyquist S.K., Mbano I.M., Miao V.N., Tzouanas C.N., Cao Y., Yousif A.S., Bals J., Hauser B.M., Feldman J., Muus C., Wadsworth M.H., 2nd, Kazer S.W., Hughes T.K., Doran B., Gatter G.J., Vukovic M., Taliaferro F., Mead B.E., Guo Z., Wang J.P., Gras D., Plaisant M., Ansari M., Angelidis I., Adler H., Sucre J.M.S., Taylor C.J., Lin B., Waghray A., Mitsialis V., Dwyer D.F., Buchheit K.M., Boyce J.A., Barrett N.A., Laidlaw T.M., Carroll S.L., Colonna L., Tkachev V., Peterson C.W., Yu A., Zheng H.B., Gideon H.P., Winchell C.G., Lin P.L., Bingle C.D., Snapper S.B., Kropski J.A., Theis F.J., Schiller H.B., Zaragosi L.E., Barbry P., Leslie A., Kiem H.P., Flynn J.L., Fortune S.M., Berger B., Finberg R.W., Kean L.S., Garber M., Schmidt A.G., Lingwood D., Shalek A.K., Ordovas-Montanes J., lung-network@humancellatlas.org, H.C.A.L.B.N.E.a. and Network, H.C.A.L.B. SARS-CoV-2 Receptor ACE2 Is an Interferon-Stimulated Gene in Human Airway Epithelial Cells and Is Detected in Specific Cell Subsets across Tissues. Cell. 2020;181(1016–1035):e19. doi: 10.1016/j.cell.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou X., Chen K., Zou J., Han P., Hao J., Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front. Med. 2020;14:185–192. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.