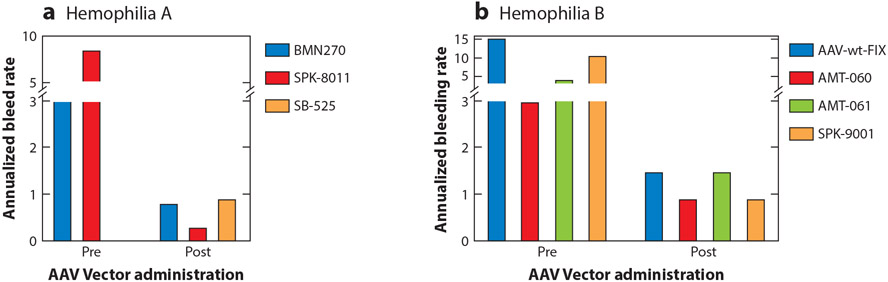
Figure 1.
Annualized bleeding rate (ABR) for participants in (a) hemophilia A and (b) hemophilia B AAV gene therapy clinical trials before and after AAV vector administration. Data were extracted as follows: BMN270 (valoctocogene roxaparvovec) represents mean ABR (n = 134 participants) (58); SPK-8011 represents mean ABR of participants who maintained expression outside an AAV capsid immune response and were followed >1 year (n = 15) (56); SB-525 (giroctocogene fitelparvovec) represents mean ABR for participants following vector administration only (n = 4) (60); AAV-wt-FIX (scAAV2/8-LP1-hFIXco) represents median reported ABR (n = 10 participants) (22, 23); AMT-060 represents mean ABR (n = 10 participants) (25); AMT-061 (etranacogene dezaparvovec) represents mean ABR of all bleeding events (n = 54 participants) (103); SPK-9001 (fidanacogene elaparvovec) represents mean ABR (n = 10 participants) (27).