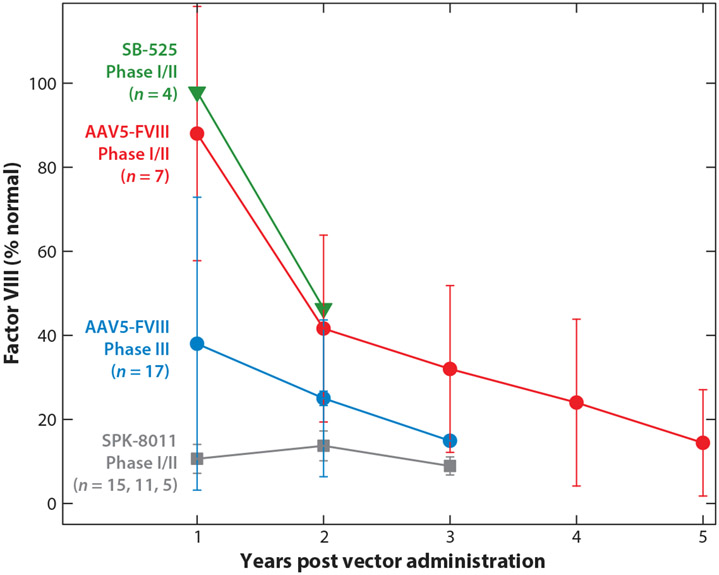
Figure 3.
Multiyear factor VIII (FVIII) activity after AAV gene therapy. Data were extracted from publicly available publications or abstracts (56-58, 86-88). Points represent median FVIII levels (except for SB-525, where only mean values were available), and error bars are the interquartile range. Data are from cohorts that received AAV vectors doses of 3 × 1013, 6 × 1013, and 0.5–2 × 1012 vg/kg of SB-525, AAV5-FVIII, and SPK-8011, respectively. Two of the participants who received SPK-8011 lost all FVIII expression due to a capsid cellular immune response within 3 months post vector administration and are not included. Plotted SPK-8011 participant data represent n = 15 at year 1, n = 11 at year 2, and n = 5 at year 3, reflecting duration of available follow-up data. FVIII activity was determined by one-stage assay or converted from a chromogenic assay result using a correction factor of 1.6 (56, 61, 104) for AAV5-FVIII results.