Abstract
Objective:
Cardioplegia and cardiopulmonary bypass (CP/CPB) dysregulate coronary vasomotor tone, which can be further affected by common comorbidities in patients undergoing cardiac surgery. This study investigates differences in coronary myogenic tone and vasomotor responses to phenylephrine before and after CP/CPB based on hypertension (HTN) history.
Methods:
Coronary arterioles before and after CP/CPB were dissected from atrial tissue samples in patients with no HTN (NH), well-controlled HTN (WC), or uncontrolled HTN (UC), as determined by documented history of HTN, anti-hypertensive agent use, and clinical blood pressure measurements averaged over one year. Myogenic tone in response to stepwise increases in intraluminal pressure was studied between pressure steps. Microvascular reactivity in response to phenylephrine was assessed via vessel myography. Protein expression was measured with immunoblotting.
Results:
Coronary myogenic tone was significantly increased in the UC group compared to NH and WC groups pre-CP/CPB at higher intraluminal pressures, and post-CP/CPB across all intraluminal pressures (p<0.05). Contractile responses to phenylephrine were significantly enhanced in patients in the UC group compared to the WC group pre-CP/CPB, and in the UC group compared to the NH and WC groups post-CP/CPB (p<0.05). There were no differences in myogenic tone or phenylephrine-induced reactivity between NH and WC groups (p>0.05). There was increased expression of p-PKCα in the UC group post-CPB compared to pre-CPB, and increased p-ERK1/2 in the UC compared to NH group post-CPB (p<0.05).
Conclusions:
Uncontrolled HTN is associated with increased coronary myogenic tone and vasoconstrictive response to phenylephrine that persists after CP/CPB.
Keywords: hypertension, coronary, microvascular, myogenic tone, cardiopulmonary bypass
INTRODUCTION:
Cardioplegia and cardiopulmonary bypass (CP/CPB) are known to dysregulate microvascular function in the peripheral and coronary circulation, which can contribute to systemic blood pressure dysregulation and myocardial malperfusion respectively.1–3 Coronary microvascular dysfunction in this setting includes altered responses to extrinsic pharmacological vasomotor agents, as well as changes in myogenic tone, or the intrinsic ability of vascular smooth muscle to regulate resistance and perfusion in the setting of systemic blood pressure changes.2,4,5 Coronary myogenic tone is regulated by several factors including changes in expression and phosphorylation i.e. activation of ERK1/2, and PKCα, both serine-threonine protein kinases which are altered with CP/CPB to drive changes in coronary tone. 6,7
Further, the effects of CP/CPB on the coronary microcirculation can be compounded in the setting of common co-morbidities affecting patients undergoing cardiac surgery. We and others have previously demonstrated the deleterious effects of CP/CPB in the setting of uncontrolled diabetes in the coronary microvasculature.4,8,9 However, the effects of other co-morbidities on the coronary microcirculation in the setting of CP/CPB have been inadequately investigated.
An important mediator of coronary microvascular injury is hypertension, which is co-morbid in at least 70–80% of patients undergoing cardiac surgery.10,11 Hypertension has been shown to cause endothelial injury via multiple mechanisms including increased oxidative stress, activation of PKC pathways, decreased shear stress, and microvascular remodeling.12 Hypertension has also been shown to dysregulate vascular myogenic tone.13,14 Further, hypertension is associated with increased sympathetic tone and may be mediated by α-adrenergic stimulation.15 Phenylephrine is an important α-adrenergic agonist used in clinical practice, and coronary constriction responses to phenylephrine have been shown to be diminished after CP/CPB, an effect that may be mediated by alterations in PKC signaling.4
Recent guidelines suggest that anti-hypertensive agents should be titrated for a goal systolic blood pressure (SBP) less than 130 mmHg and diastolic blood pressure less than 80 mmHg in patients with cardiovascular disease.16 However, studies are lacking on the effects of blood pressure control per these guidelines on the coronary microvascular tone, which has direct implications on myocardial perfusion. Therefore, the goal of this study is to investigate differences in coronary myogenic tone and vasomotor responses to phenylephrine before and after CP/CPB in patients with no hypertension, well-controlled hypertension, and uncontrolled hypertension. We hypothesized that coronary myogenic tone and contractile response to phenylephrine would be increased in the setting of uncontrolled hypertension, as mediated by alterations in ERK1/2 and PKC signaling pathways.
METHODS:
Tissue Collection
Right atrial tissue was harvested before and after CPB from patients undergoing cardiac surgery with CP/CPB. Two samples were taken from the right atrial appendage using a double-purse-string technique with 3–0 polypropylene sutures. The first tissue sample (“pre-CPB”) was collected before initiation of CPB during placement of the venous cannula into the right atrium. During collection, the superior suture was tightened to secure the cannula, while the inferior suture remained loose, thereby allowing the atrial tissue between the sutures to be exposed to cardioplegia solution, the effects of CPB, and the effects of reperfusion. The second atrial sample (“post-CPB”) was collected between the two purse-string sutures after cross clamp removal and termination of CPB, but before protamine administration. Atrial samples were immediately frozen in liquid nitrogen for immunoblotting or stored in cold Krebs buffer for in-vitro microvascular reactivity studies.
Groups were defined based on documented history of hypertension (HTN), use of prescription anti-hypertensive agents, and average clinic systolic blood pressure (SBP) measurements over one year prior to cardiac surgery. The non-hypertensive group (NH) was defined as patients with no documented active HTN, no use of prescription anti-hypertensive agents, and an average SBP less than 130 mmHg. The well-controlled HTN (WC) group was defined as patients with a documented history of HTN, use of prescription anti-hypertensive agents, and an average SBP less than 130 mmHg. The uncontrolled HTN (UC) group was defined as patients with a documented history of HTN, with or without use of prescription anti-hypertensive agents, and an average SBP greater than 130 mmHg. Patients undergoing an emergent procedure were excluded. Regarding anti-hypertensive agents, it is the practice of our institution to instruct patients to hold angiotensin-converting enzyme inhibitors and angiotensin receptor blockers 48 hours prior to surgery, and to continue beta blockers and calcium channel blockers through the morning of surgery. Prescription anti-hypertensive agent use was documented as of the patient’s most recent history and physical documentation.
All procedures were approved by the Institutional Review Board (IRB) of Rhode Island Hospital, Alpert Medical School of Brown University (IRB #004410, 03/10/10), and informed consent was obtained from all enrolled patients prior to tissue collection and involvement in the study as required by the IRB. (Figure 5) (Video Abstract)
Figure 5: Localization of p-PKCα and p-ERK1/2 in coronary microvessels.
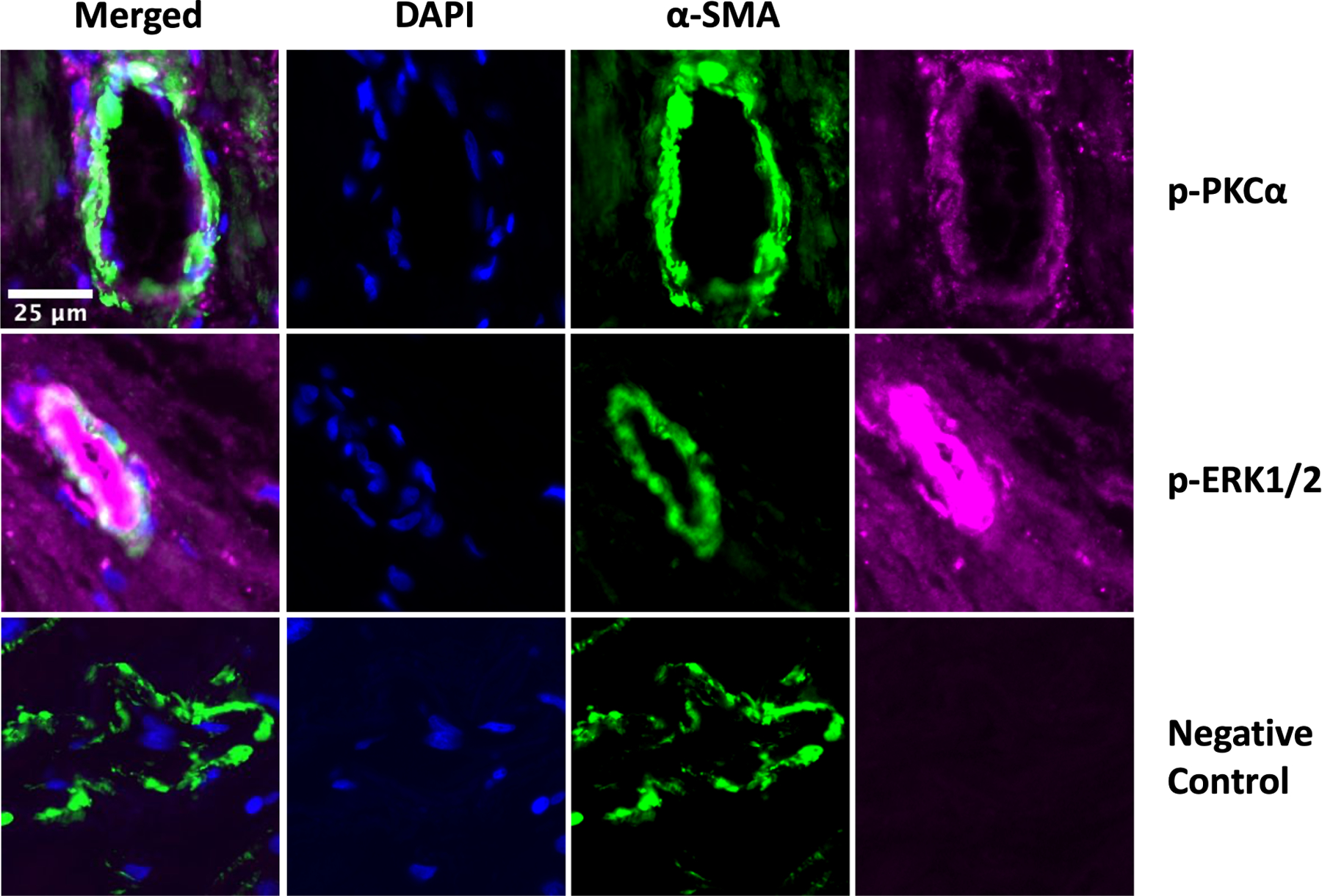
Immunofluorescent staining of human atrial tissue sections demonstrated expression of phosphorylated protein kinase C alpha (p-PKCα, purple) and phosphorylated extracellular signal-regulated kinase 1/2 (p-ERK1/2, purple) on the coronary microvessel walls as stained by α-smooth muscle actin (α-SMA, green). Nuclei stained with DAPI (blue). Negative control stained only with α-SMA showed low level of background fluorescence without p-PKCα and p-ERK1/2 signals. Images obtained at 20X magnification.
In-vitro Coronary Microvascular Studies
Coronary arterioles (80–180μm internal diameter) were harvested out of human atrial tissue from NH (n=5), WC (n=8), and UC (n=13) groups, and dissected using a dissecting microscope. Depending on tissue availability, some patient samples were adequate for use in both myogenic tone and phenylephrine treatment studies, while others were adequate for use in only one study. Microvessels were placed in a microvessel chamber containing circulating warm (37 degrees Celsius) and oxygenated (95% oxygen and 5% carbon dioxide) Krebs buffer solution, cannulated with dual glass micropipettes (30–80μm in diameter), and secured in place with 10–0 nylon monofilament sutures. The microvessel image was projected onto a monitor using an inverted microscope (40–200x, Olympus CK2, Olympus Optical) connected to a video camera. An electronic dimension analyzer was used to measure the internal luminal diameter.
For myogenic tone studies, NH (n=5), WC (n=8), and UC (n=10) coronary arteriole internal diameters were measured with stepwise increases in intraluminal pressure of 20–120 mmHg, an ex-vivo model of mean arterial pressure. At each pressure, the vessel was allowed to reach a steady-state diameter for 3 minutes prior to measurement. Internal diameters of microvessels were normalized to the microvessel diameter after treatment with papaverine. Increased myogenic tone with stepwise increases in intraluminal pressure is reflected by decreased arteriolar internal diameter. (Supplementary Figure 1)
For phenylephrine treatment studies, NH (n=4), WC (n=5), and UC (n=9) coronary arterioles were pressurized to 40 mmHg in a no-flow state by using a burette manometer filled with Krebs buffer solution. The microvessels were allowed to bathe in the Krebs buffer-containing microvessel chamber for at least 30 minutes, then phenylephrine was applied to the vessel in increasing concentrations (10−9-10−4M), and in-vitro contractile responses were assessed via vessel myography. (Video)
Immunoblotting
Coronary atrial tissue samples from four patients per NH, WC, and UC groups were dissected and cleaned of connective tissues and solubilized in RIPA buffer. Total protein (37μg) was fractionated on a 4–12% Bis-Tris gel (ThermoFisher Scientific), transferred to a nitrocellulose membrane (ThermoFisher Scientific, Waltham, MA), and membranes were incubated overnight at 4 degrees Celsius with 1:1000 dilutions of individual rabbit polyclonal primary antibodies to protein kinase C alpha (PKCα), phosphorylated PKCα (p-PKCα), extracellular signal-regulated kinase 1/2 (ERK1/2), phosphorylated ERK1/2 (p-ERK1/2), α-adrenergic 1A, and α-adrenergic 1B receptors (Abcam, Cambridge, UK). The membranes were then washed and incubated with goat polyclonal anti-rabbit or anti-mouse secondary antibody for one hour at room temperature. Membranes were then washed and processed for chemiluminescent detection and captured with a digital camera system (Bio-Rad ChemiDoc MP, Life Science, Hercules, CA). All membranes were probed with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Cell Signaling, Danvers, MA) to correct for loading error. Densitometric analysis of band intensity was performed using NIH Image J software.
Immunofluorescence
Immunofluorescence studies were performed as previously described.4 Briefly, paraffin-embedded atrial tissue sections from three patients were de-paraffinized, antigen unmasked with sodium citrate, blocked with 3% BSA, and incubated with primary antibodies to α-smooth muscle actin (α-SMA) and p-PKCα, p-ERK1/2, or no additional antibody which served as the negative control. Sections were then incubated with Alexa-fluor tagged secondary antibodies (Abcam, Cambridge, UK). Images were captured at 20X magnification with an Olympus VS200 Slide Scanner.
Data Analysis
Baseline myogenic tone initially measured as diameter in µm in response to stepwise increases in intraluminal pressure were normalized as previously discussed. Microvessel responses to phenylephrine are expressed as percent constriction compared to baseline. Microvascular reactivity data were analyzed using 2-way repeated measures ANOVA followed by Tukey’s multiple comparison’s test if indicated. Western blot data were analyzed using paired t tests with Bonferroni corrections to compare densitometry of samples before and after CPB, and unpaired t tests with Bonferroni corrections to compare densitometry of samples from NH vs WC vs UC patients pre-bypass or post-bypass. Data are reported as mean and standard deviation (SD). Probability values <0.05 were considered significant.
RESULTS:
Patient Characteristics
The patient characteristics are listed in Table 1. Tissue samples from a total of 37 patients were studied, with 9 patients in the NH group (5 for microvessel studies, 4 for immunoblotting), 12 patients in the WC group (8 for microvessel studies, 4 for immunoblotting), and 16 patients in the UC group (13 for microvessel studies, 4 for immunoblotting – one patient’s tissue sample availability adequate for both microvessel and immunoblotting studies). Mean age was 63 ± 10 years old in the NH group, 65 ± 10 years old in the WC group, and 68 ± 7 years old in the UC group. Gender distribution was 8 males and 1 female in the NH group, 11 males and 1 female in the WC group, and 14 males and 2 females in the UC group. Patients from each group underwent a variety of cardiac surgical procedures with the majority being coronary artery bypass grafting. The average clinic systolic BP in mmHg was 119 ± 10 in the NH group, 117 ± 9 in the WC group, and 138 ± 6 in the UC group. Classes of anti-hypertensive drugs prescribed to patients as of their most recent pre-operative history and physical documentation are listed in Table 1. One patient in the NH group was prescribed a beta blocker for an indication other than hypertension. Of note, all patients in Table 1 who were not previously on beta blockers were started on beta blockers pre-operatively for cardioprotection.
Table 1:
Baseline characteristics.
| Baseline Characteristics | No HTN (n=9) | Controlled HTN (n=12) | Uncontrolled HTN (n=16) |
|---|---|---|---|
| Age – yr. | 63 ± 10 | 65 ± 10 | 68 ± 7 |
| Male/Female – n (%) | 8 (89%) | 11 (92%) | 14 (88%) |
| CABG only – n (%) | 7 (78%) | 6 (50%) | 12 (75%) |
| Valve surgery only – n (%) | 1 (11%) | 3 (25%) | 3 (19%) |
| CABG + valve surgery - n (%) | 1 (11%) | 3 (25%) | 1 (6%) |
| HbA1c (%) | 6.2 ± 1.6 | 6.9 ± 1.3 | 6.2 ± 0.8 |
| BMI (kg/m2) | 27.6 ± 5.7 | 32.4 ± 7.0 | 31.2 ± 4.6 |
| CPB Time (min) | 92 ± 47 | 141 ± 57 | 118 ± 61 |
| Cross Clamp Time (min) | 71 ± 40 | 107 ± 43 | 95 ± 51 |
| Systolic BP (mmHg) | 119 ± 10 | 117 ± 9 | 138 ± 6 |
| Diastolic BP (mmHg) | 72 ± 5 | 69 ± 6 | 77 ± 7 |
| Anti-hypertensive drug class | |||
| ACEi – n (%) | 0 (0%) | 5 (42%) | 6 (38%) |
| ARB – n (%) | 0 (0%) | 2 (17%) | 6 (38%) |
| Calcium Channel Blocker – n (%) | 0 (0%) | 3 (25%) | 5 (31%) |
| Beta Blocker – n (%) | 1 (11%) | 10 (83%) | 9 (56%) |
| Thiazide – n (%) | 0 (0%) | 3 (25%) | 4 (25%) |
| Loop Diuretic – n (%) | 0 (0%) | 3 (25%) | 0 (0%) |
Categorical variables presented as no. (%); Continuous variables presented as mean ± standard deviation; Abbreviations: HTN = hypertension; CABG = coronary artery bypass surgery; AVR = aortic valve replacement; MVR = mitral valve replacement; BP = blood pressure; HbA1c = hemoglobin A1c; BMI = body mass index; CPB = cardiopulmonary bypass; ACEi = angiotensin converting enzyme inhibitor; ARB = angiotensin II receptor blocker
Microvascular Reactivity
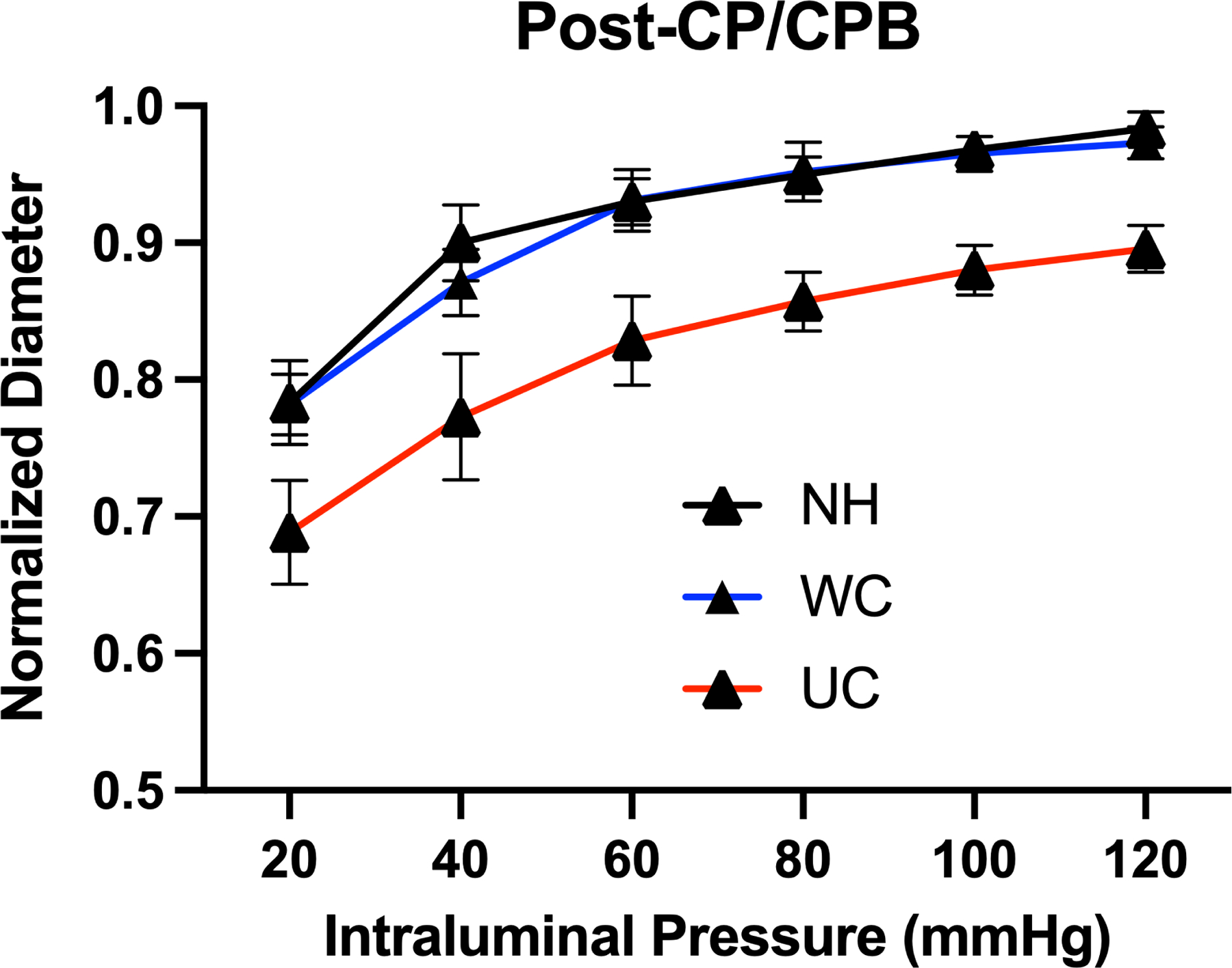
Prior to cardiopulmonary bypass, there was a significant increase in myogenic tone in the UC group compared to patients in the NH and WC groups at intraluminal coronary pressures of 120 mmHg (p<0.05) and compared to patients in the WC group at pressures of 100 mmHg (p<0.05). After cardiopulmonary bypass, there was a significant increase in myogenic tone in patients in the UC group compared to patients with NH and WC groups across all intraluminal pressures (p<0.05). There was no significant difference in myogenic tone between the NH and WC groups (Figure 1). Cardiopulmonary bypass was associated with a significant decrease in myogenic tone among patients in all groups (Figure 1).
Figure 1: Coronary Myogenic Tone Before and After Cardioplegia / Cardiopulmonary Bypass.
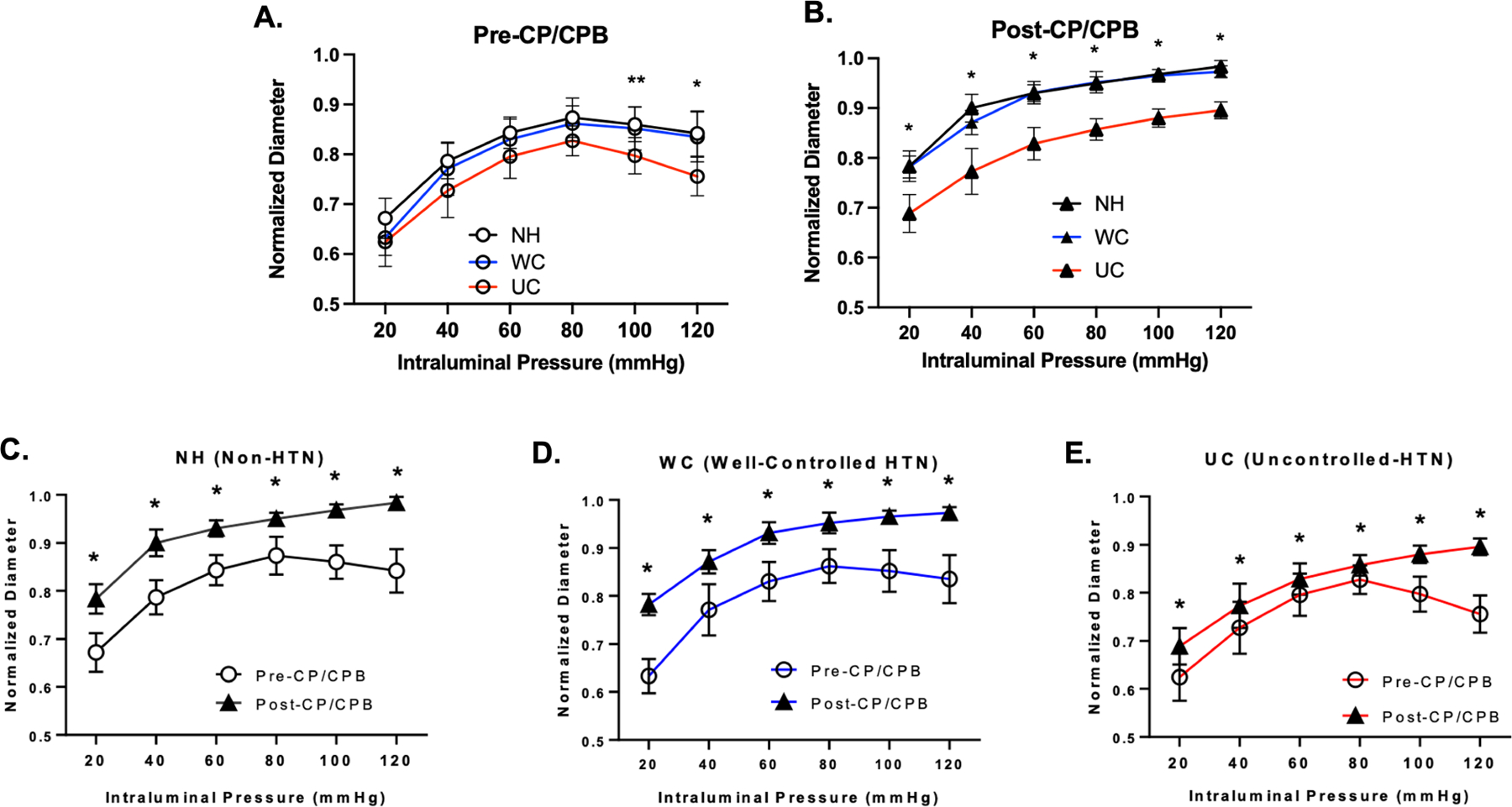
Coronary arteriolar normalized diameter response to intraluminal pressure (20 – 120 mmHg) in patients with no hypertension (NH, n=5), well-controlled hypertension (WC, n=8), and uncontrolled hypertension (UC, n=10) (A) before (pre-) cardioplegia/cardiopulmonary bypass (CP/CPB), and (B) after (post-) CP/CPB. Data also presented as pre- and post-CP/CPB responses subdivided by patients with (C) no hypertension (NH), (D) well-controlled hypertension (WC), and (E) uncontrolled hypertension (UC). Data plotted as mean ± SD; * p < 0.05 UC vs. WC and UC vs. NH; ** p < 0.05 UC vs WC.
Pre-CP/CPB contractile response to phenylephrine was significantly enhanced in patients within the UC group compared to patients with WC group at higher doses of phenylephrine (p<0.05). Post-CP/CPB contractile responses to phenylephrine were enhanced to a lesser degree in patients with poorly controlled HTN compared to patients in the WC and NH groups only at higher doses of phenylephrine (p<0.05). There were no significant differences in phenylephrine-induced vasomotor tone between patients in the NH and WC (p>0.05) (Figure 2). All three groups had significantly decreased contractile responses to phenylephrine at higher doses post-CP/CPB compared to pre-CP/CPB. (Figure 2)
Figure 2: Dose-dependent constriction in response to phenylephrine in in-vitro coronary arterioles.
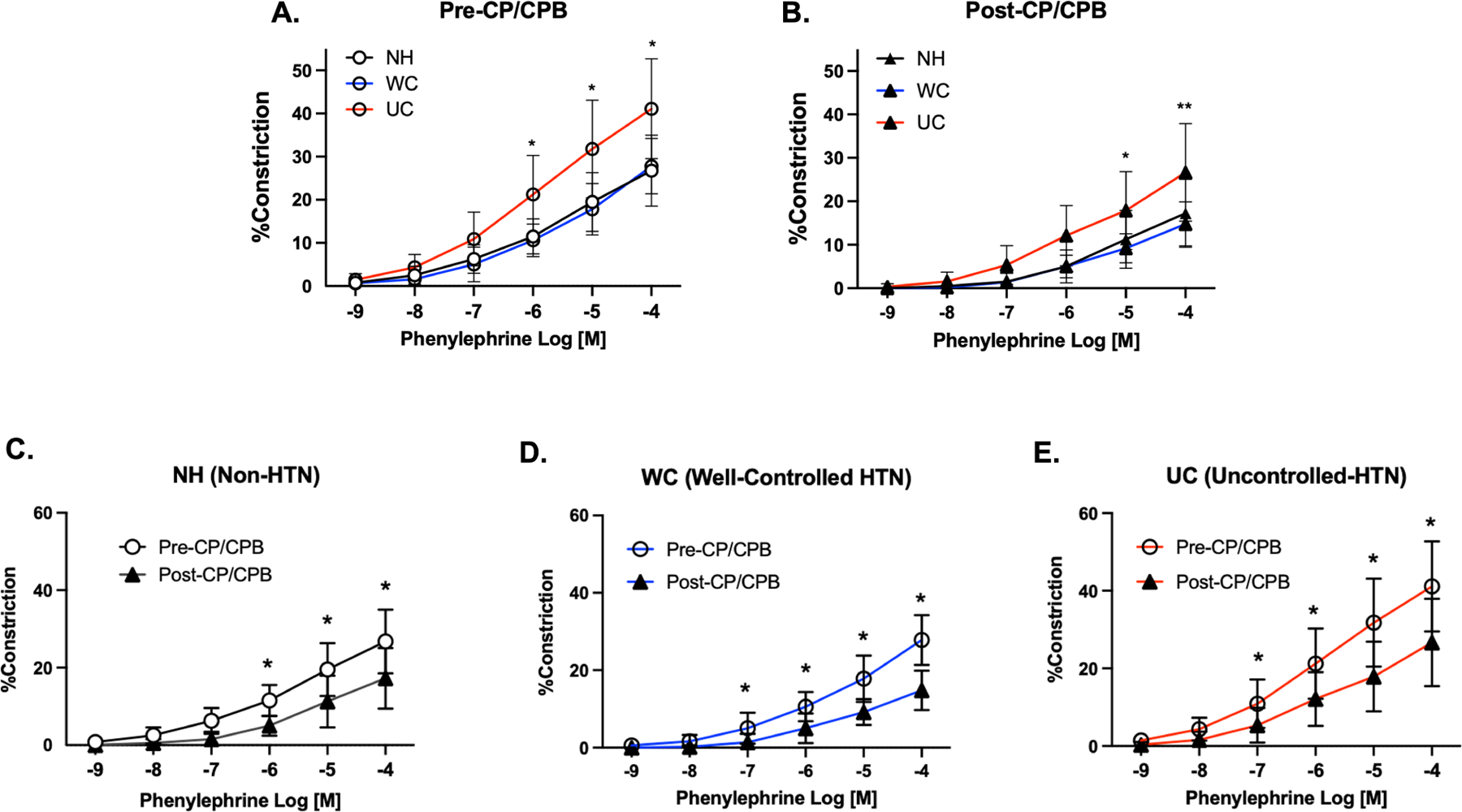
coronary arteriolar constriction response to phenylephrine (10−9-10−4 M) in non-HTN (NH, n=4), well-controlled HTN (WC, n=5), and uncontrolled HTN (UC, n=9) patients (A) before (pre-) cardioplegia/cardiopulmonary bypass (CP/CPB), and (B) after (post-) CP/CPB. Data also presented as pre- and post-CP/CPB responses subdivided by patients with (C) no hypertension (NH), (D) well-controlled hypertension (WC), and (E) uncontrolled hypertension (UC). Data plotted as mean ± SD; * p < 0.05 UC vs. WC; ** p < 0.05 UC vs. WC and UC vs. NH.
Protein Expression
Immunoblot staining showed a significant increase in expression of the p-PKCα in the UC group post-CPB compared to pre-CPB (p=0.003). There was a trend towards increased ratio of p-PKCα and PKCα in the UC group post-CPB compared to pre-CPB that did not reach significance (p=0.09).
There was significantly increased expression of p-ERK1/2 in the UC group post-CP/CPB compared to the NH group post-CP/CPB (p=0.032), and increased ratio of p-ERK1/2 and ERK1/2 in the UC group post-CP/CPB compared to the NH group post-CP/CPB (p=0.036).
There was also a trend toward decreased p-ERK1/2 and the ratio of p-ERK1/2 and ERK1/2 post-CP/CPB compared to pre-CP/CPB in the NH and WC groups, and increased p-ERK1/2 and the ratio of p-ERK1/2 and ERK1/2 post-CP/CPB compared to pre-CP/CPB in the UC group, but these did not approach significance (Figure 3).
Figure 3: Protein expression of PKCα and ERK1/2.
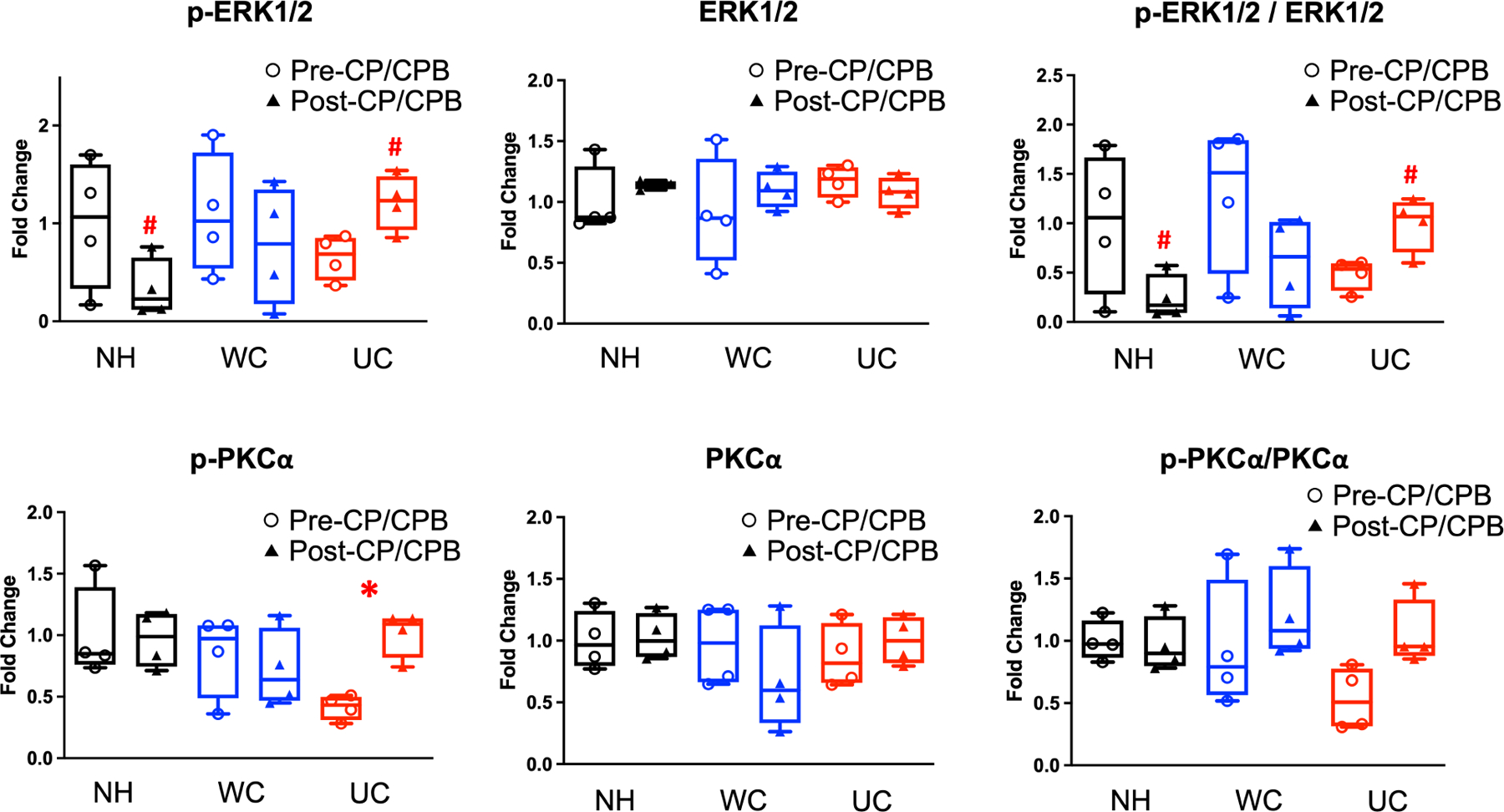
Western blot protein expression analysis of phosphorylated and total protein kinase C alpha (p-PKCα, PKCα) and extracellular signal-regulated kinases 1 & 2 (p-ERK 1/2, ERK 1/2) in non-HTN (NH), well-controlled HTN (WC), and uncontrolled HTN (UC) patients before (pre-) and after (post-) cardioplegia/cardiopulmonary bypass (CP/CPB). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a loading control. Data presented as fold changes in optical density compared to average optical density in NH group. Blot images are provided in Supplementary Figure 2. n = 4/group. Upper and lower borders of box represent upper and lower quartiles, middle horizontal line represents median, upper and lower whiskers represent maximum and minimum values of non-outliers. *p<0.05 pre-CPB vs post-CPB. #p<0.05 NH post-CPB vs UC post-CPB.
There were no significant differences in expression of α−1A adrenergic receptor or α−1B adrenergic receptors in the NH, WC, or UC groups at baseline or after CP/CPB (Figure 4).
Figure 4: Protein expression of α−1 Adrenergic Receptors.
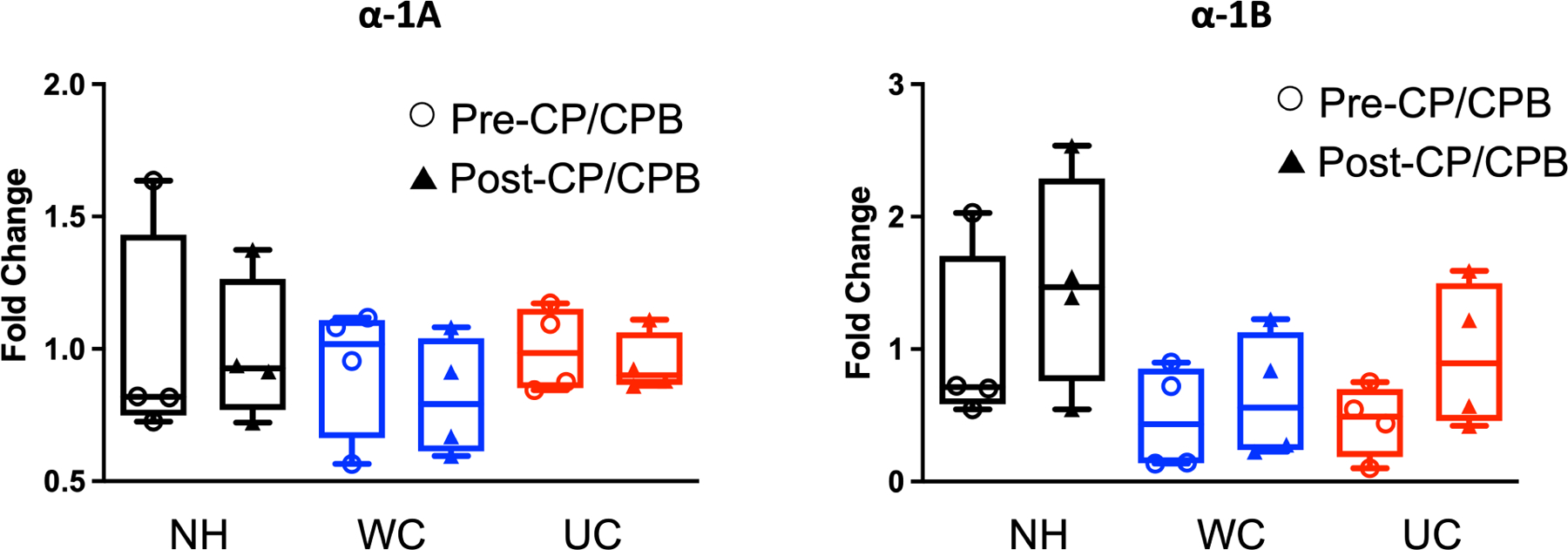
Western blot protein expression analysis of α−1A and α−1B adrenergic receptor subtypes in non-HTN (NH), well-controlled HTN (WC), and uncontrolled HTN (UC) patients before (pre-) and after (post-) cardioplegia/cardiopulmonary bypass (CP/CPB). Data presented as fold changes in optical density compared to average optical density in NH group. Blot images are provided in supplementary figure 2. n = 4/group. Upper and lower borders of box represent upper and lower quartiles, middle horizontal line represents median, upper and lower whiskers represent maximum and minimum values of non-outliers.
Protein Localization
Immunofluorescent staining of tissue sections displayed a signal for p-PKCα and p-ERK1/2 on the coronary microvessel walls as stained by α-SMA. (Figure 5)
DISCUSSION:
In this study, we demonstrated that the loss of coronary myogenic tone that occurs following CP/CPB is less pronounced in patients with uncontrolled hypertension compared to patients with no hypertension and well-controlled hypertension. Further, coronary arteriolar contractile responses to phenylephrine are increased in patients with uncontrolled hypertension before and after CP/CPB. Increased myogenic tone in patients with uncontrolled hypertension was associated with increased expression of p-PKCα post-CP/CPB compared to pre-CP/CPB, and with increased expression of p-ERK1/2 post-CP/CPB in patients with uncontrolled hypertension compared to normotension. There were no differences in protein expression of α-adrenergic receptors 1A and 1B based on hypertension history, suggesting that differences in contractile responses to phenylephrine in the setting of co-morbid hypertension may be due to altered post-receptor molecular signaling pathways.
The coronary microcirculation, which is critical in the regulation of myocardial perfusion, is comprised of vessels <200μm in diameter and includes capillaries and arterioles. Arteriolar smooth muscle has an intrinsic ability to constrict in response to increased vascular intraluminal pressure, a phenomenon described as myogenic tone.17 Myogenic tone plays an important role in autoregulation of myocardial and cerebral perfusion in response to changes in systemic blood pressure.5,14 Therefore, factors that dysregulate coronary arteriolar myogenic tone may directly affect myocardial perfusion.
Cardioplegia and cardiopulmonary bypass are known to disrupt coronary microcirculatory function, leading to impaired endothelial-dependent arteriolar dilation and impaired myogenic tone.3,5 In the present study, we have redemonstrated that CP/CPB is associated with decreased myogenic tone among all patient groups. Hypertension also affects myogenic tone, and treating hypertension has been shown to reverse effects in the cerebral microcirculation.14 Interestingly, uncontrolled hypertension, when compared to well-controlled hypertension or normotension, was associated with increased coronary myogenic tone at higher intraluminal pressures at baseline, but after CP/CPB, this effect was apparent across all intraluminal pressures. The clinical consequences of this increased myogenic tone following CP/CPB in the uncontrolled HTN group compared to well-controlled and non-hypertensive patients are not yet clear, given that poor myogenic tone may lead to myocardial edema and dysfunction, while elevated tone may reduce perfusion and contribute to vasospasm.18 Therefore, the possibility that a blunted decrease in myogenic tone following CP/CPB among patients with uncontrolled HTN could be cardioprotective in this particular setting may warrant further investigation. However, baseline increases in coronary myogenic tone among patients with poorly controlled hypertension, particularly in the setting of coronary artery disease, could have deleterious effects on myocardial perfusion.
Coronary myogenic tone is regulated by many factors, including changes in calcium signaling, ERK1/2 signaling, and PKC activity, particularly the PKCα isoform.6,7 We investigated whether changes in protein expression of PKCα and ERK1/2, and in their phosphorylated active forms, may account for increases in coronary myogenic tone among patients with poorly controlled hypertension. We hypothesized that these proteins would be the main drivers of changes in myogenic tone after CP/CPB. After CP/CPB, there was increased expression of p-PKCα in patients with uncontrolled hypertension compared to pre-CP/CPB, which could account for the blunted loss of myogenic tone following CP/CPB in this group. Further, phosphorylation of ERK1/2 appears to play a role in the differences in myogenic tone between groups, with increased expression of p-ERK1/2 and increased ratio of p-ERK1/2 and ERK1/2 in patients with uncontrolled HTN compared to normotension after CP/CPB, a finding which was also consistent with differences in myogenic tone between these groups. Our findings suggest that phosphorylation of PKCα and ERK1/2 may contribute to increased coronary myogenic tone after CP/CPB in patients with uncontrolled hypertension relative to patients with normotension. However, despite trends toward increased expression of p-ERK1/2 and in the ratio of p-ERK1 and ERK1/2 in patients with uncontrolled hypertension compared to well-controlled hypertension, there were no significant changes to explain differences in myogenic tone between these two groups. Therefore, we cannot exclude changes in other parts of the signaling pathway contributing to the alterations that we observed in myogenic tone.
Additionally, coronary tone may be regulated by a number of other extrinsic factors, including neurohormonal substances.19 One important drug used clinically is phenylephrine, which is a selective α−1 adrenergic receptor agonist. α−1 adrenergic activation is an important mediator of coronary arteriolar vasoconstriction in the setting of endothelial dysfunction.20 The results of this study suggest that phenylephrine causes increased coronary microcirculatory vasoconstriction in patients with a history poorly controlled hypertension when compared to patients with well-controlled hypertension. Though the vasoconstrictive response to phenylephrine is blunted after CPB in all groups, patients with uncontrolled hypertension still have increased coronary vasoconstrictive responses to higher doses of phenylephrine when compared to other groups. Increased coronary vasoconstriction in patients with uncontrolled hypertension could have important clinical consequences such as reduced coronary flow and myocardial perfusion in the post-operative setting in this patient population. These findings also underscore the importance of adequate pre-operative management of co-morbidities in patients undergoing cardiac surgery, a theme that has been previously investigated in patients with controlled versus uncontrolled diabetes.8 Changes in coronary responses to phenylephrine based on hypertension status were not explained by differences in baseline or post-CPB levels of adrenergic α−1A or α−1B receptors, both activated by phenylephrine to induce vasoconstriction. Alterations in PKCα signaling are also known to impair phenylephrine-mediated adrenergic signaling,2 and the increased expression of phosphorylated PKCα in patients with uncontrolled HTN may contribute to augmented contractile responses to phenylephrine compared to other groups. However, further studies are warranted to investigate additional mechanisms by which phenylephrine induced vasoconstriction is altered in patients with poorly controlled hypertension. Future studies from our lab will also investigate whether hypertension status alters vasomotor responses to other clinically relevant vasomodulators such as nitroprusside and vasopressin in the setting of CP/CPB.
An important consideration is whether the differences in coronary microvascular tone noted in our study among hypertension groups before and after CPB have clinically important implications. First, hypertension is associated with diastolic heart failure, rarefaction of the microvasculature in the heart and other organs,21 and increased myogenic tone under baseline conditions.13,14,17 In addition, elderly patients have less potential to repair vascular injury.22 Secondly, the findings of this study may have implications in vasoplegic syndrome, manifesting as decreased systemic vascular resistance and hypotension after cardiac surgery with CP/CPB. While peripheral and coronary microvascular function may correlate,23 microvascular changes that result in systemic vasoplegia would be better investigated in non-coronary peripheral tissue beds. The current study’s focus on coronary microvascular tone is, in contrast, most directly related to myocardial tissue perfusion. Therefore, we would exercise caution in extrapolating these findings to physiologic changes responsible for systemic vasoplegia, and studies in this area on peripheral vascular beds are planned by our group to better address how hypertension status and CP/CPB affect peripheral vascular tone.
A limitation to the present study includes low sample size, particularly in the western blot analysis of four per group, in order to compare protein expression across a total of six different groups with a single experimental design. These small group sizes may not be powered enough to detect important differences in expression of proteins involved in coronary myogenic tone. Additionally, there is wider heterogeneity in this human study of coronary microvascular tone compared to animal studies that have more controlled parameters. We attempted to characterize important baseline characteristic distributions that were relevant to coronary microvascular disease, including gender, BMI, and HbA1c, but there are likely other unaccounted for co-morbidities and variables that may influence coronary microvascular tone in these patients. Finally, the study was subject to recall bias given the assignment of patients into categories based on pre-defined HTN status.
CONCLUSION:
In conclusion, poorly controlled hypertension is associated with a blunted decrease in coronary myogenic tone after cardiopulmonary bypass and increased vasoconstrictive response to phenylephrine that persists after cardiopulmonary bypass, when compared to patients with no hypertension or well-controlled hypertension (Figure 6). The mechanisms and clinical implications of these changes and the changes that occur in other vascular beds warrant further investigation.
Figure 6: Graphical Abstract.
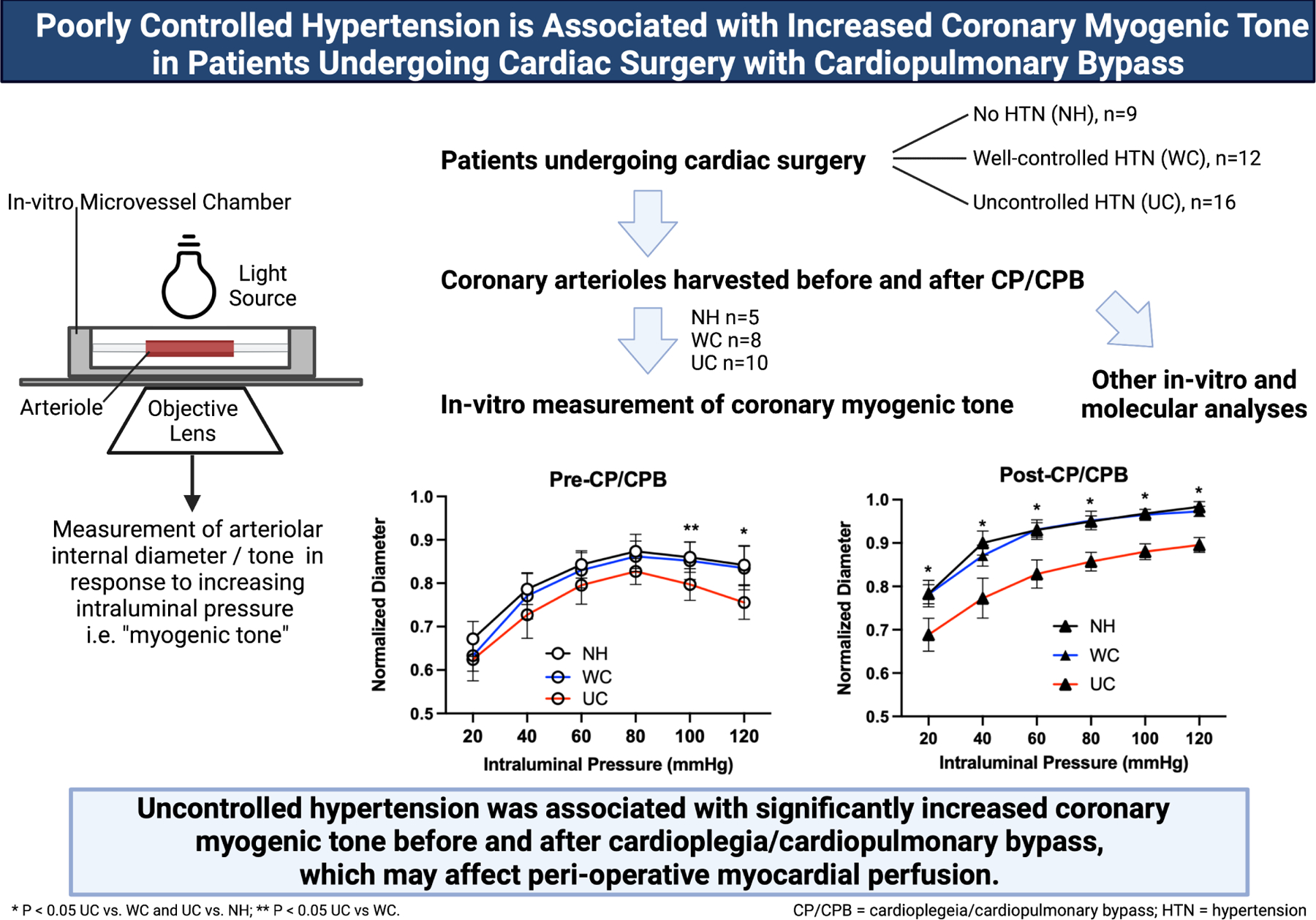
Patients who underwent cardiac surgery with cardioplegia/cardiopulmonary bypass (CP/CPB) were divided into three groups: no hypertension (HTN) (NH, n=9), well-controlled HTN (WC, n=12), uncontrolled HTN (UC, n=16). Atrial tissue before and after CP/CPB was harvested. Tissue samples were allocated to coronary myogenic tone studies (NH n=5, WC n=8, UC n=10), or other in-vitro and molecular analyses, specifically phenylephrine treatment studies and western blotting. For myogenic tone studies, coronary arterioles were dissected out. In-vitro measurement of coronary myogenic tone was carried out by measurement of arteriolar diameter in response to stepwise increases in intraluminal pressure. Uncontrolled hypertension was associated with increased coronary myogenic tone before and after CP/CPB.
Supplementary Material
Supplementary Figure 1: Set up for Myogenic Tone Measurements. Human coronary arterioles from harvested atrial tissue samples were dissected using a dissecting microscope. Arterioles were placed in a microvessel chamber containing circulating warm, oxygenated Krebs buffer. Arterioles were cannulated with dual glass micropipettes and secured with 10–0 nylon monofilament suture. Vessels were pressurized to 20–120 mmHg using a pressure controller connected to the glass micropipette. With an inverted microscope connected to a video camera, the vessel image was projected onto a television monitor. An electronic dimension analyzer was used to measure the internal lumen diameter.
Supplementary Figure 2: Western blot images. Complete western blot images for expression of protein kinase C alpha (PKCα), phosphorylated PKCα (p-PKCα), extracellular signal-regulated kinase 1/2 (ERK1/2), phosphorylated ERK1/2 (p-ERK1/2), α−1A adrenergic receptor (α−1A), and α−1B adrenergic receptor (α−1B). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a loading control. Given quantity of samples, western blot was run across two membranes as indicated by space in between images. Two samples were replicated in the same location across both membranes as indicated by * in order to correct for intermembrane differences.
In-vitro microvessels require an external heat exchanger, pressure controller, circulating oxygenated Krebs buffer solution, and an inverted microscope connected to a video camera, which images the microvessel within a chamber containing the warm buffer solution. The image projects to a monitor, and an electronic dimension analyzer is used to measure internal lumen diameter. For myogenic tone studies, coronary arteriole internal diameters are measured with stepwise increases in intraluminal pressure. Pressure measurements are recorded with a digital chart recorder, and intraluminal diameter measurements are plotted to correspond pressure to diameter changes.
CENTRAL PICTURE:
Coronary Myogenic Tone After Cardioplegia / Cardiopulmonary Bypass. *p<0.05.
CENTRAL MESSAGE:
Patients with uncontrolled hypertension have increased coronary myogenic tone before and after cardiopulmonary bypass compared to patients with no hypertension or well-controlled hypertension.
PERSPECTIVE STATEMENT:
Cardiopulmonary bypass (CPB) dysregulates coronary microvascular tone which affects myocardial perfusion. Patients undergoing cardiac surgery often have co-morbid hypertension, which also dysregulates microvascular tone. These effects may depend on pre-operative blood pressure control. We found that uncontrolled hypertension is associated with increased coronary myogenic tone before and after CPB.
ACKNOWLEDGEMENTS:
We would like to thank all the nurses, physician assistants and perfusionists working in cardiac surgery at Rhode Island Hospital for collecting the tissue samples and recording patient characteristics. We would also like to thank the nurses and physician assistants in the Division of Cardiac Surgery of Rhode Island Hospital for collecting patient consent forms.
Funding:
This study was supported by the National Heart, Lung, and Blood Institute (NHLBI) 1F32HL160063-01 (S.A.S.); R01HL46716 and R01HL128831-01A1 (F.W.S.), 1R01HL127072-01A1 and 1R01HL136347-01 (J.F.)
GLOSSARY OF ABBREVIATIONS
- CP/CPB
cardioplegia and cardiopulmonary bypass
- ERK1/2
extracellular signal-regulated kinase 1/2
- pERK1/2
phosphorylated extracellular signal-regulated kinase 1/2
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- HTN
hypertension
- NH
no hypertension group
- PKCα
protein kinase C alpha
- pPKC
phosphorylated protein kinase C alpha
- SBP
systolic blood pressure
- UC
uncontrolled hypertension group
- WC
well controlled hypertension group
Footnotes
Disclosures: None
Presentation: The American Heart Association Scientific Sessions 2021, Nov. 13–15, Chicago, IL
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ruel M, Khan TA, Voisine P, Bianchi C, Sellke FW. Vasomotor dysfunction after cardiac surgery. Eur J Cardio-Thorac Surg Off J Eur Assoc Cardio-Thorac Surg 2004. Nov;26(5):1002–14. [DOI] [PubMed] [Google Scholar]
- 2.Sodha NR, Feng J, Clements RT, Bianchi C, Boodhwani M, Ramlawi B, et al. Protein kinase C alpha modulates microvascular reactivity in the human coronary and skeletal microcirculation. Surgery 2007. Aug;142(2):243–52. [DOI] [PubMed] [Google Scholar]
- 3.Sellke FW, Shafique T, Schoen FJ, Weintraub RM. Impaired endothelium-dependent coronary microvascular relaxation after cold potassium cardioplegia and reperfusion. J Thorac Cardiovasc Surg 1993. Jan;105(1):52–8. [PubMed] [Google Scholar]
- 4.Sellke N, Gordon C, Lawandy I, Gorvitovskaia AY, Scrimgeour LA, Fingleton JG, et al. Impaired coronary contraction to phenylephrine after cardioplegic arrest in diabetic patients. J Surg Res 2018. Oct;230:80–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang SY, Friedman M, Franklin A, Sellke FW. Myogenic reactivity of coronary resistance arteries after cardiopulmonary bypass and hyperkalemic cardioplegia. Circulation 1995. Sep 15;92(6):1590–6. [DOI] [PubMed] [Google Scholar]
- 6.Amin AH, Elmageed ZYA, Partyka M, Matrougui K. Mechanisms of Myogenic Tone of Coronary Arteriole: Role of Down Stream Signaling of the EGFR Tyrosine Kinase. Microvasc Res 2011. Jan;81(1):135–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dessy C, Matsuda N, Hulvershorn J, Sougnez CL, Sellke FW, Morgan KG. Evidence for involvement of the PKC-alpha isoform in myogenic contractions of the coronary microcirculation. Am J Physiol Heart Circ Physiol 2000. Sep;279(3):H916–923. [DOI] [PubMed] [Google Scholar]
- 8.Feng J, Liu Y, Chu LM, Singh AK, Dobrilovic N, Fingleton JG, et al. Changes in microvascular reactivity after cardiopulmonary bypass in patients with poorly controlled versus controlled diabetes. Circulation 2012. Sep 11;126(11 Suppl 1):S73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feng J, Chu LM, Dobrilovic N, Liu Y, Singh AK, Sellke FW. Decreased coronary microvascular reactivity after cardioplegic arrest in patients with uncontrolled diabetes mellitus. Surgery 2012. Aug;152(2):262–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shah RM, Zhang Q, Chatterjee S, Cheema F, Loor G, Lemaire SA, et al. Incidence, Cost, and Risk Factors for Readmission After Coronary Artery Bypass Grafting. Ann Thorac Surg 2019. Jun;107(6):1782–9. [DOI] [PubMed] [Google Scholar]
- 11.Terada T, Johnson JA, Norris C, Padwal R, Qiu W, Sharma AM, et al. Severe Obesity Is Associated With Increased Risk of Early Complications and Extended Length of Stay Following Coronary Artery Bypass Grafting Surgery. J Am Heart Assoc Cardiovasc Cerebrovasc Dis 2016. Jun 1;5(6):e003282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sabe SA, Feng J, Sellke FW, Abid MR. Mechanisms and Clinical Implications of Endothelium-dependent Vasomotor Dysfunction in Coronary Microvasculature. Am J Physiol Heart Circ Physiol 2022. Mar 25; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia SR, Izzard AS, Heagerty AM, Bund SJ. Myogenic tone in coronary arteries from spontaneously hypertensive rats. J Vasc Res 1997. Apr;34(2):109–16. [DOI] [PubMed] [Google Scholar]
- 14.Pires PW, Jackson WF, Dorrance AM. Regulation of myogenic tone and structure of parenchymal arterioles by hypertension and the mineralocorticoid receptor. Am J Physiol - Heart Circ Physiol 2015. Jul 1;309(1):H127–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Michel MC, Brodde OE, Insel PA. Peripheral adrenergic receptors in hypertension. Hypertens Dallas Tex 1979 1990. Aug;16(2):107–20. [DOI] [PubMed] [Google Scholar]
- 16.Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertens Dallas Tex 1979 2018. Jun;71(6):1269–324. [DOI] [PubMed] [Google Scholar]
- 17.Jackson WF. Myogenic Tone in Peripheral Resistance Arteries and Arterioles: The Pressure Is On! Front Physiol 2021;12:699517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khan TA, Bianchi C, Ruel M, Voisine P, Li J, Liddicoat JR, et al. Mitogen-Activated Protein Kinase Inhibition and Cardioplegia-Cardiopulmonary Bypass Reduce Coronary Myogenic Tone. Circulation 2003. Sep 9;108(10_suppl_1):II–348. [DOI] [PubMed] [Google Scholar]
- 19.Goodwill AG, Dick GM, Kiel AM, Tune JD. Regulation of Coronary Blood Flow. Compr Physiol 2017. Mar 16;7(2):321–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heusch G, Baumgart D, Camici P, Chilian W, Gregorini L, Hess O, et al. alpha-adrenergic coronary vasoconstriction and myocardial ischemia in humans. Circulation 2000. Feb 15;101(6):689–94. [DOI] [PubMed] [Google Scholar]
- 21.Hoenig MR, Bianchi C, Rosenzweig A, Sellke FW. The cardiac microvasculature in hypertension, cardiac hypertrophy and diastolic heart failure. Curr Vasc Pharmacol 2008. Oct;6(4):292–300. [DOI] [PubMed] [Google Scholar]
- 22.Hoenig MR, Bianchi C, Rosenzweig A, Sellke FW. Decreased vascular repair and neovascularization with ageing: mechanisms and clinical relevance with an emphasis on hypoxia-inducible factor-1. Curr Mol Med 2008. Dec;8(8):754–67. [DOI] [PubMed] [Google Scholar]
- 23.Al-Badri A, Kim JH, Liu C, Mehta PK, Quyyumi AA. Peripheral Microvascular Function Reflects Coronary Vascular Function. Arterioscler Thromb Vasc Biol 2019. Jul;39(7):1492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Set up for Myogenic Tone Measurements. Human coronary arterioles from harvested atrial tissue samples were dissected using a dissecting microscope. Arterioles were placed in a microvessel chamber containing circulating warm, oxygenated Krebs buffer. Arterioles were cannulated with dual glass micropipettes and secured with 10–0 nylon monofilament suture. Vessels were pressurized to 20–120 mmHg using a pressure controller connected to the glass micropipette. With an inverted microscope connected to a video camera, the vessel image was projected onto a television monitor. An electronic dimension analyzer was used to measure the internal lumen diameter.
Supplementary Figure 2: Western blot images. Complete western blot images for expression of protein kinase C alpha (PKCα), phosphorylated PKCα (p-PKCα), extracellular signal-regulated kinase 1/2 (ERK1/2), phosphorylated ERK1/2 (p-ERK1/2), α−1A adrenergic receptor (α−1A), and α−1B adrenergic receptor (α−1B). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a loading control. Given quantity of samples, western blot was run across two membranes as indicated by space in between images. Two samples were replicated in the same location across both membranes as indicated by * in order to correct for intermembrane differences.
In-vitro microvessels require an external heat exchanger, pressure controller, circulating oxygenated Krebs buffer solution, and an inverted microscope connected to a video camera, which images the microvessel within a chamber containing the warm buffer solution. The image projects to a monitor, and an electronic dimension analyzer is used to measure internal lumen diameter. For myogenic tone studies, coronary arteriole internal diameters are measured with stepwise increases in intraluminal pressure. Pressure measurements are recorded with a digital chart recorder, and intraluminal diameter measurements are plotted to correspond pressure to diameter changes.


