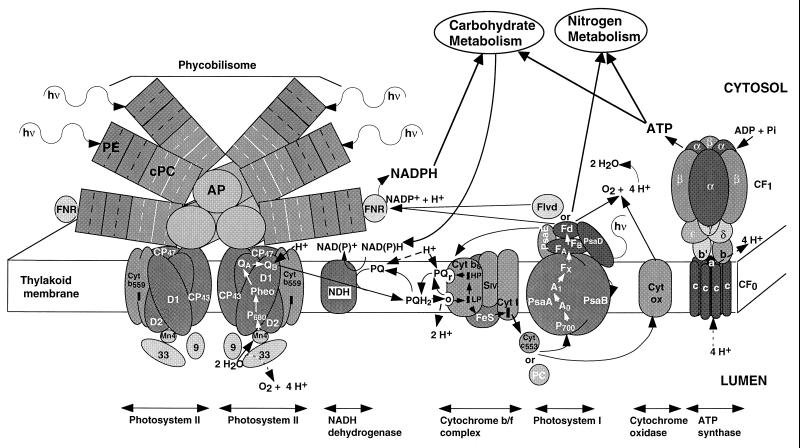
FIG. 1.
Cyanobacterial thylakoid electron transport. This schematic diagram is based on data primarily from Synechocystis sp. strain PCC 6701, which contains phycoerythrin, phycocyanin, and allophycocyanin pigment proteins in the phycobilisomes. The phycobilisomes move rapidly along the surface of the thylakoid membranes (96), so the phycobilisome-PS II dimer complex is transient. Excitation absorbed by the phycobilisome can reach either PS II or PS I, particularly in cells in state II. This excitation flow may involve specialized subunits of the phycobilisome core (not shown here). The composition and organization of the phycobilisome rods and core is variable in different cyanobacteria; the three-cylinder core and six-peripheral rod configuration is common, but in Synechococcus sp. strain PCC 7942 the core contains only two cylinders. The PS I-PS II stoichiometry is usually higher than 1:1; in the Synechococcus cells used for most experiments described in later figures, the ratio was 2 to 3 PS I/PS II. There are multiple interacting and flexible paths of electron flow including linear flow from water to NADPH; several possible cyclic pathways around subsections of the transport system; pseudocyclic flows from water with electron donation back to oxygen; and respiratory flows of electrons derived from reserve molecules. Some possible flows are indicated by black arrows. The donor-acceptor stoichiometries of electron transfers are not shown, but various redox centres carry different numbers of electrons, from 1 (e.g., PC, cytochrome c553, Fd, and Flvd), 2 [e.g., PQ and NAD(P)H] and even 4 (the Mn complex of PS II). The redox reactions are reversible depending upon the oxidation-reduction status of the acceptor-donor pair and the local proton concentration, so that in some cases the indicated direction of electron flow could be reversed. Proton uptake and transmembrane transport are indicated by dashed arrows; other proton translocation pathways may also exist. The plastoquinone-plastoquinol pool can be reduced by electrons from PS II, from an NAD(P)H dehydrogenase(s) (NDH) whose composition and substrate specifities vary between strains, and from the “r” site of the cytochrome bf complex. Plastoquinone reduction by NDH is the entry point for electrons derived from respiration. The various plastoquinone reductions involve proton uptake from the stroma, while oxidation of a plastoquinol at the ‘o’ site of the cytochrome bf complex releases two protons to the lumen. As indicated, electron and ATP flow to carbohydrate and nitrogen metabolism can have strong and rapid effects on thylakoid function, while electrons derived from carbohydrate reserves also enter the thylakoid system and influence photosynthetic function. During ATP synthesis, protons enter a channel from the lumen formed by the a subunit of Cf0, and their exit to the cytosol is coupled to rotation of the ring of c subunits through directed diffusion (37). The c-ring rotation drives rotation of the γ subunit of CF1 within the α3β3 ring (117), which in turn drives a sequence of conformational changes in three identical ATP/ADP binding sites. The changes in binding site lead to phosphorylation of ADP and expulsion of ATP from the site. The stoichiometry is 1 ATP/4H+ passing through the complex (146). Abbreviations: hν, photons of visible light; PE, phycoerythrin α3β3 trimers; CPC, phycocyanin α3β3 trimers; AP, allophycocyanin rods of the phycobilisome core, composed partly of α3β3 trimer disks along with other related phycobilin-binding proteins; D1 and D2, core polypeptide dimer of PS II which binds the redox cofactors; Cyt b559, cytochrome b559 in the PS II core; Mn4, manganese cluster of the oxygen evolving complex; 9 and 33, 9- and 33-kDa subunits of the oxygen evolving complex of PS II; CP43 and CP47, 43- and 47-kDa chlorophyll protein complexes associated with the PS II core; P680, dimeric chlorophyll center which is photooxidized in PS II; Pheo, pheophytin primary electron acceptor of PS II; QA, the quinone secondary electron acceptor of PS II; QB, a plastoquinone bound to PS II which accepts two electrons from QA and equilibrates with the thylakoidal plastoquinone-plastoquinol pool; NDH, NAD(P)H dehydrogenase (in various strains there are different forms of the complex with differing activities and specificities for NADH or NADPH); Cyt b6, a cytochrome containing both low- and high-potential heme centers which are involved in a Q-cycle electron flow from plastoquinol bound to the o site to plastoquinone bound to the “r” site (this cycle results in proton translocation); SIV, subunit IV of the cytochrome bf complex; FeS, an iron-sulfur redox center; PC, plastocyanin, a copper-containing luminal single-electron transport protein; Cyt c553, cytochrome c553, a heme-containing luminal single-electron transport protein (plastocyanin and cytochrome c553 can be reciprocally regulated in response to copper and iron availability); PsaA and PsaB, related chlorophyll binding proteins which form the core of PS I; P700, the chlorophyll which is photooxidized in PS I; A0, A1, FX, FA, FB, bound redox intermediates of PS I; Flvd, flavodoxin (a flavin protein which is a cytosolic mobile single electron carrier that can accept electrons from PS I and that can substitute for ferredoxin, particularly under low-iron conditions); Fd, ferredoxin (an iron protein which is a cytosolic mobile single-electron carrier that can accept electrons from PS I and can transfer the electrons to NADPH or participate directly in some biosynthetic reactions, particularly in nitrogen metabolism); FNR, ferredoxin/flavodoxin NADPH oxidoreductase; Cyt ox, the cytochrome oxidase complex involved in respiratory electron transport (it can also withdraw electrons from photosynthetic electron transport, particularly under excess light); α to γ, subunits of the CF1 complex of ATP synthase; a to c, subunits of the CF0 complex of ATP synthase. Modified and redrawn from reference 19 with permission of the publisher.