Abstract
The present study aimed to compare the clinical effects of vitamin E and vitamin D on a rat model of dextran sulfate sodium (DSS)-induced ulcerative colitis (UC), and to elucidate the underlying mechanisms associated with changes in the levels of cytokines. After successful establishment of the rat model of DSS-induced UC, prednisolone (1 mg/kg), vitamin D (50 ng) and vitamin E (6, 30 and 150 IU/kg) were orally administered for 1 week. The pharmacodynamics were evaluated by a daily combination of clinical observation (CO) scores, histopathological evaluations and assessment of molecular markers of inflammation. Administration of vitamin D, vitamin E (30 and 150 IU/kg), prednisolone, and the combination of vitamin D and vitamin E resulted in a decrease in CO scores. The severity of inflammation of the colon was markedly alleviated in the treatment groups compared with that in the untreated DSS group according to the results of histopathological examination; however, they showed different inhibitory effects on the levels of some cytokines. In conclusion, the present results indicated that oral administration of vitamin E could promote recovery of DSS-induced UC by the inhibition of proinflammatory cytokines, and that its underlying mechanism may differ from that of vitamin D and glucocorticoid drugs.
Keywords: vitamin E, inflammation, ulcerative colitis, cytokines, colon
Introduction
Inflammatory bowel disease (IBD), including Crohn's disease (CD) and ulcerative colitis (UC), represents a group of intestinal disorders that cause prolonged inflammation of the digestive tract. IBD affects ~0.1% of the Western population and has contributed to an increased risk of morbidity (1-3). A growing incidence of IBD has also been reported worldwide (4,5). According to a recent report, the annual incidence rate per 100,000 people in Asia is 0.5 to 21.6, in Central America and South America it is 0.4 to 3.0, in Europe it is 0 to 21.3, in North America it is 2.4 to 15.4, and in Oceania it is 5.2 to 6.8(5).
The clinical symptoms of IBD include diarrhea, repeated rectal bleeding, abdominal pain, body weight (BW) loss and severe malnutrition (1-3). Although the etiology of IBD remains unclear, an increasing body of evidence has revealed a multifactorial disease associated with susceptible genes, intestinal microbiota and environmental factors, leading to the dysregulation of both adaptive and innate immune systems. This can be characterized by the abnormal activation of intestinal immune cells, followed by the release of a range of inflammatory factors. It is well accepted that numerous cytokines, such as interleukin (IL)-1β (6-8), IL-6(6), IL-12 (9,10), IL-18 (9,11), tumor necrosis factor-α (TNF-α) (7,12,13) and interferon-γ (IFN-γ) (8), are involved in the pathogenesis of both types of IBD and are considered indicators of therapeutic efficacy. Corticosteroids, aminosalicylate, some biological agents and immunosuppressive agents are mainly used for the treatment of IBD; however, they cannot fundamentally prevent the disease recurring (14,15).
Notably, patients with IBD mainly experience a loss of appetite due to nausea, vomiting, abdominal pain and diarrhea, and folate deficiency has classically been associated with anemia in these patients (16-19). Thus, vitamins, such as vitamin D, vitamin B6, vitamin B12 and vitamin C, are occasionally recommended for patients with IBD (20-24). Several studies have shown that vitamin D supplementation can prevent and ameliorate symptoms of IBD (25-27). Animals with either vitamin D deficiency or defective vitamin D receptors are susceptible to developing IBD (28-30). Furthermore, vitamin D can change the composition of the intestinal flora through regulation of the expression of antibacterial peptides, in addition to immune regulation (31). Phillips et al (32) demonstrated that vitamin D may provide some protection against increased mitochondrial dysfunction and inflammation in the placenta of obese women. In addition, Hahn et al (33) indicated that long-term vitamin D supplementation may be effective for reducing the incidence of autoimmune diseases. During the coronavirus 2019 (COVID-19) pandemic, vitamin D was found to be efficacious in attenuating the release of inflammatory cytokines after viral infection through its antimicrobial and anti-inflammatory properties (34). Moreover, moderate daily doses of vitamin D have been shown to slow down the progression of Parkinson's disease and prevent COVID-19 infection in the elderly (34,35). In addition, it has been reported that although genetic differences can lead to different benefits of vitamin D in different populations, 40-60 ng/ml (100-150 mmol/l) of serum 25-hydroxyvitamin D is the concentration that achieves the best overall health benefits (36). In infants and young children, vitamin D supplementation can also reduce the incidence of childhood asthma and other allergic diseases (37). These functions maintain the integrity of intestinal mucosa as a surface barrier and repair mucosal permeability (38). By inhibiting the activation of cellular immunity and cytotoxic T cells, vitamin D supplementation can also adjust the immune response. Therefore, vitamin D deficiency can endanger the mucosal barrier, resulting in mucosal injury and increasing the risk of IBD (19,22). Dextran sodium sulfate (DSS)-induced colitis in rats is a model resembling human UC. Other studies have shown that adequate vitamin D supplementation can be effective in preventing respiratory diseases. Epithelial cells, dendritic cells (DCs) and macrophages in the lungs are efficacious in producing active vitamin D, which enhances the production of cytokines with anti-inflammatory functions and promotes the production of anti-viral peptides (39). Previous evidence has suggested that oxidative stress is an important component in the pathophysiology of IBD (40-44), and some antioxidants have exhibited protective and healing effects against DSS-induced UC in rats (45).
Vitamin E is a well-accepted, relatively safe antioxidant in cellular membranes and can protect membrane lipids from peroxidation. A previous study revealed that vitamin E has anti-inflammatory effects following inflammation in the lung (46). Thus, vitamin E could exhibit a strong effect on UC due to its anti-inflammatory activity and high antioxidant capacity. It has been shown that vitamin D and vitamin E can also have a preventive effect for the treatment of hair loss. Vitamin D maintains serum levels of calcium and phosphorus, in addition to its anti-inflammatory and immunological effects. Notably, vitamin D regulates keratinocyte growth and differentiation through the nuclear vitamin D receptor to prevent hair loss and type II rickets (47). Vitamin E can also prevent hair loss and baldness by maintaining the oxidant/antioxidant balance (47). However, Carrier et al (48) reported that vitamin E supplementation increased clinical observation (CO) scores from colonic inflammation, and that it did not affect oxidative stress, thus indicating that vitamin E may have an unclear mechanism of reducing inflammation. By contrast, another study reported that vitamin E showed a dual-effect of anti-inflammatory and antioxidant activities on acetic acid-induced UC in rats (49).
Various animal models of IBD have been developed in the last decade, which are valuable and indispensable tools for evaluating different therapeutic options for IBD (6,7,9). The DSS-induced UC model in rats has some advantages compared with other animal models of UC, and is a widely used model resembling human UC. Rats exposed to DSS in drinking water develop inflammation in the large intestine and exhibit signs such as diarrhea, anemia, BW loss and histological inflammation, including inflammatory cell infiltration, muscularis mucosae erosion and ulcers, with increased levels of pro-inflammatory cytokines (e.g., IFN-γ, TNF-α, IL-1, IL-6 and IL-12) (40,45,50).
Whether vitamin E plays a protective role, similar to vitamin D, in conditions such as UC still needs to be elucidated, although vitamin E deficiency was previously found in patients with IBD (48,50). Thus, the present study aimed to systemically evaluate the anti-inflammatory effects of vitamin E on a rat model of DSS-induced UC and to compare the effects with those of vitamin D.
Materials and methods
Chemicals and reagents
All reagents and chemicals used in the present study were purchased from the companies listed below, unless otherwise stated. DSS salt (M.W., 36,000-50,000) was purchased from MP Biomedicals LLC. DL-α-Tocopheryl acetate (all-rac-α-Tocopheryl acetate Vitamin E acetate; M.W., 472.74 g/mol) was obtained from MilliporeSigma. Vitamin D (1α, 25-dihydroxycholecalciferol; M.W., 416.6 g/mol) was purchased from Roche Diagnostics (Shanghai) Co., Ltd. Paraformaldehyde was obtained from Sinopharm Chemical Reagent Co., Ltd., with sodium chloride injection (0.9%) from Shijiazhuang No. 4 Pharmaceutical Co., Ltd.). Radio-immunoprecipitation (RIPA) assay lysis buffer was purchased from Qingdao Jisskang Biotechology Co., Ltd.) and prednisolone acetate from Huazhong Pharmaceutical Co., Ltd.).
Animals and treatment
Male Wistar rats (age, 5 weeks; n=120; n=12-24 rats/group; weight, 200±10 g) were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. The rats were housed in a vivarium under a 12-h light/dark cycle at constant temperature (22±2˚C) and humidity (50-60%), with free access to food and water, in accordance with the principles of the Good Lab Practice (GLP) guidelines presented by the National Beijing Center for Drug Safety Evaluation and Research (51-53). Animals were acclimated to the laboratory for 1 week before starting experiments.
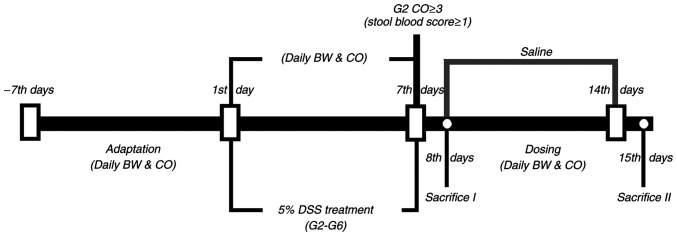
After the adaptation period, the rats were divided into eight groups [(G1, G2, G3, G4, G5a, G5b, G5c and G6], and the doses are summarized in Table I. UC in male Wistar rats was induced by adding 5% (w/v) DSS to the drinking water from day 1 to day 7, whereas rats in the control group (G1) received only tap water (Fig. 1). After successfully establishing the rat model of DSS-induced UC according to weight loss and stool examination, rats in the G4 to G6 groups received different doses of vitamin E and vitamin D (Table I) from day 8 to day 14, and were sacrificed on day 15 for pathological examination. Meanwhile, half of the rats in the G1 and G2 groups were sacrificed on day 8 for pathological detection. The remaining rats in the G1 and G2 groups received saline from day 8 to day 14, and were sacrificed on day 15 for pathological examination. The rats were anesthetized by intraperitoneal injection of 5% pentobarbital sodium (50 mg/kg) and blood was collected from the abdominal aorta following anesthesia; mice were sacrificed by exsanguination.
Table I.
Groups of rats analyzed in the present study.
| Group name | Treatment | Number of rats | Route of administration | Dosage of administration |
|---|---|---|---|---|
| G1 | Control (vehicle) | 24 | Drinking water | |
| G2 | DSS (Day 1-7) | 24 | Dissolved in drinking water | 5% |
| G3 | DSSa (Day 1-7) + prednisolone (Day 8-14) | 12 | Orally administered (Day 8-14) | 1.0 mg/kg |
| G4 | DSSa (Day 1-7) + vitamin D (Day 8-14) | 12 | Orally administered (Day 8-14) | 50 ng |
| G5a | DSSa (Day 1-7) + vitamin E (low dose) (Day 8-14) | 12 | Orally administered (Day 8-14) | 6 IU/kg |
| G5b | DSSa (Day 1-7) + vitamin E (medium dose) (Day 8-14) | 12 | Orally administered (Day 8-14) | 30 IU/kg |
| G5c | DSSa (Day 1-7) + vitamin E (high dose) (Day 8-14) | 12 | Orally administered (Day 8-14) | 150 IU/kg |
| G6 | DSSa (Day 1-7) + vitamin D + vitamin E (Day 8-14) | 12 | Orally administered (Day 8-14) | 50 ng + 30 IU/kg |
aDissolved in drinking water (5%). DSS, dextran sodium sulfate.
Figure 1.
Schematic diagram of the experimental process and study design. DSS, dextran sodium sulfate. Saline, G1-G6 were administrated with saline from day 7 to day 14; Sacrifice I, half of the rats in G1 and G2; Sacrifice II, all of the remaining rats; CO, clinical observation; BW, body weight; G1, control group; G2, 5% DSS group; G3, prednisolone group; G4, vitamin D group; G5a-c, vitamin E (low, medium and high) groups; G6, vitamin D + vitamin E group; DSS, dextran sodium sulfate.
Study design and clinical observation (CO) score
For each rat in the six groups, BW and CO score were recorded daily. Half of the rats in both the G1 and G2 groups were sacrificed on day 8 to collect the blood and tissue samples for histopathological examination, and cytokine measurement. The remaining rats also underwent these tests, when they were culled on day 15. The experimental design is shown in Fig. 1.
For the CO score, both stool score and stool blood score were recorded separately and combined to generate a total CO score with a maximum score of 5. Stool scoring was performed as follows: 0, normal; 1, moist/sticky stool; 2, stool in or around anus; 3, diarrhea. Stool blood scoring was carried out as follows: 0, no blood; 1, evidence of blood in stool or around anus; 2, severe bleeding. For CO scoring, the experiment was conducted according to a GLP standard. No stool images are provided in this study.
For the colon weight/length ratio (%), half of the rats in groups G1 and G2 were sacrificed on day 8 and the colon weight/length ratio (%) was measured. The remaining rats in groups G1-G6 were sacrificed on day 15 and the colon weight/length ratio (%) was measured.
Gross and histopathological evaluation of hematoxylin and eosin (H&E)-stained colonic tissue
For the histopathological examination, the proximal colon (1 cm) was fixed in 10% formaldehyde for 24 h at room temperature. The tissues were then dehydrated in a graded series of alcohol [70% (2 h), 80% (2 h), 90% (2 h), 95% alcohol (2 h), anhydrous alcohol (1 h)]; then cleared with xylene for 1 h. The tissues were then infiltrated with paraffin at 58-60˚C for 2 h and the paraffin blocks were sliced into ~5-µm sections at 45˚C and mounted onto glass slides. The sections were dewaxed with xylene for 15 min and were incubated with alcohol of different concentrations (100, 95, 80 and 70% alcohol) to remove paraffin. H&E staining was performed using a standard staining procedure; the sections were incubated in hematoxylin solution for 5 min, then incubated with eosin for 2-3 min. Eight randomly selected fields (magnification, x100) in each slide were observed under a light microscope (Olympus BX43; Olympus Corporation) as described previously (54).
Enzyme-linked immunosorbent assay (ELISA) of colonic cytokines
Briefly, colon tissues were homogenized in 1 ml ice-cold RIPA assay lysis buffer, containing 1% protease inhibitor cocktail and 1% phosphatase inhibitor cocktail. The lysate was centrifuged at 15,000 x g for 15 min at 4˚C, and the supernatant was transferred to 96-well ELISA plates before measurement of the following inflammatory markers: IL-6 (cat. no. SEKR-0005), IL-12 (cat. no. SEKR-0057), IL-18 (cat. no. SEKR-0054), TNF-α (cat. no. SEKR-0009) and IFN-γ (cat. no. SEKR-0008) using kits according to the manufacturer's protocols.
Statistical analysis
Continuous data are presented as the mean ± standard error of the mean, whereas CO score data are expressed as the median and interquartile range. Comparisons between two groups were performed using the unpaired Student's t-test. Multiple comparisons were performed by one- or two-way analysis of variance and Bonferroni's post hoc test. For the CO scores, Kruskal-Wallis was conducted followed by Dunn's post hoc test for statistical analysis. GraphPad Prism 6.0 software (GraphPad Software, Inc.) was used to perform the statistical analysis. P<0.05 was considered to indicate a statistically significant difference.
Results
Both vitamin D and vitamin E prevent BW loss in a rat model of DSS-induced UC
DSS can cause damage to the colonic mucosal barrier, leading to gut inflammation and BW loss (40). The present study demonstrated that rats that received drinking water with 5% DSS had significantly more BW loss than those in the control group (G1) from day 4 to day 8 (Fig. 2, F=7.43, P=0.01). Rats in the vitamin D-treated group (G4), vitamin E-treated groups (G5a-c), vitamin D + vitamin E group (G6) and prednisolone group (G3) had a similar degree of BW loss as rats in G2 from day 4 to day 8 (F=2.83, P=0.998). However, rats in G4, G5a-c and G6 experienced a rapid BW recovery from day 11 to day 15 compared with those in G2 (F=6.13, P=0.01), whereas no significant difference was found in BW between G3 and G6 (F=5.97, P=0.995).
Figure 2.
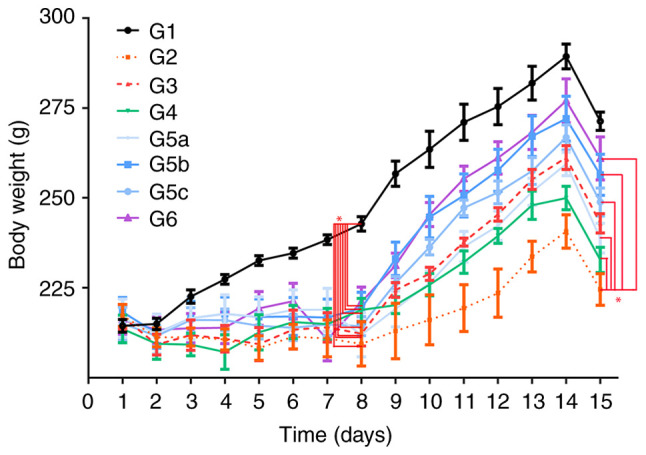
Measurements of body weight in G1, G2, G3, G4, G5a-c and G6. The body weight in each group was measured from day 1 to day 15. G1, control group; G2, 5% DSS group; G3, prednisolone group; G4, vitamin D group; G5a-c, vitamin E (low, medium and high) groups; G6, vitamin D + vitamin E group; DSS, dextran sodium sulfate. *P<0.05.
Vitamin D and vitamin E ameliorate the clinical symptoms of UC in rats
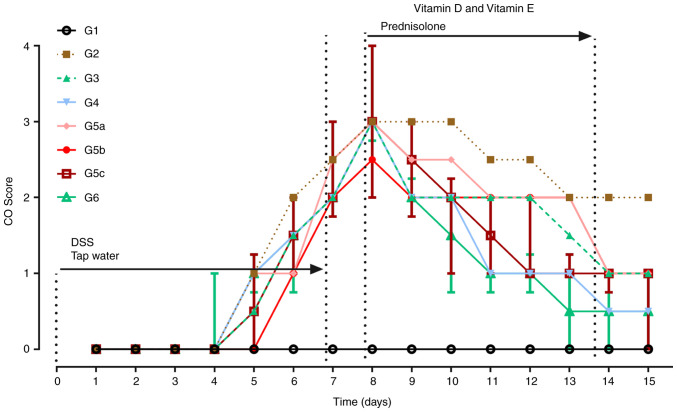
In the experiment, CO scoring was performed daily (Fig. 3). Compared with rats in G1, rats treated with DSS (G2, G3, G4, G5a-c and G6) showed obvious symptoms of diarrhea with noticeably higher CO scores from days 5 to 8 (χ2=19.28, P=0.007; Fig. 3). Rats in G3, G4, G5a-c and G6 had a lower blood score and firmer stools than those in G2 from day 9 to day 15. On day 15, CO scores in the drug-treated groups (G3, G4, G5a-c and G6) were significantly lower than those in G2 (χ2=12.77, P=0.04; Fig. 3).
Figure 3.
Changes in CO scores across the whole experiment. From day 1 to day 7, rats in G1 received drinking water, whereas rats in the other groups received water containing DSS (5%, w/v). Compared with those in G1, CO scores were significantly higher in the other groups (G2-G6) on day 8. On day 15, CO scores in G3, G4, G5c, G5b and G6 were significantly lower than those in G2, whereas there was no statistically significant difference between G2 and G5a. Data are presented as the median and interquartile. n=12 for each group. G1, control group; G2, 5% DSS group; G3, prednisolone group; G4, vitamin D group; G5a-c, vitamin E (low, medium and high) groups; G6, vitamin D + vitamin E group; CO, clinical observation; DSS, dextran sodium sulfate.
Both vitamin D and vitamin E inhibit DSS-induced colonic inflammation
To verify whether the rat model of DSS-induced UC was successfully established, half of the rats in G1 and G2 were sacrificed for histopathological examinations on day 8. Rats in G2 had more severe macroscopic inflammation than those in G1 (Fig. 4A). Consistent with macroscopic observations, the colon length in G2 on day 8 was shorter than that in G1 (Fig. 4A). On day 15, the colon length in G3 and G6 was longer than that in G2 (Fig. 4B). Compared with G2, there was no difference in colon length in G4 and G5, but there was a marked difference in colon length between G6 and G2. Although colon length in G6 was not as long as that in G3, the experimental result provided novel evidence for the treatment of UC. These findings indicated that the combination of vitamin D and vitamin E may be better than individual use.
Figure 4.
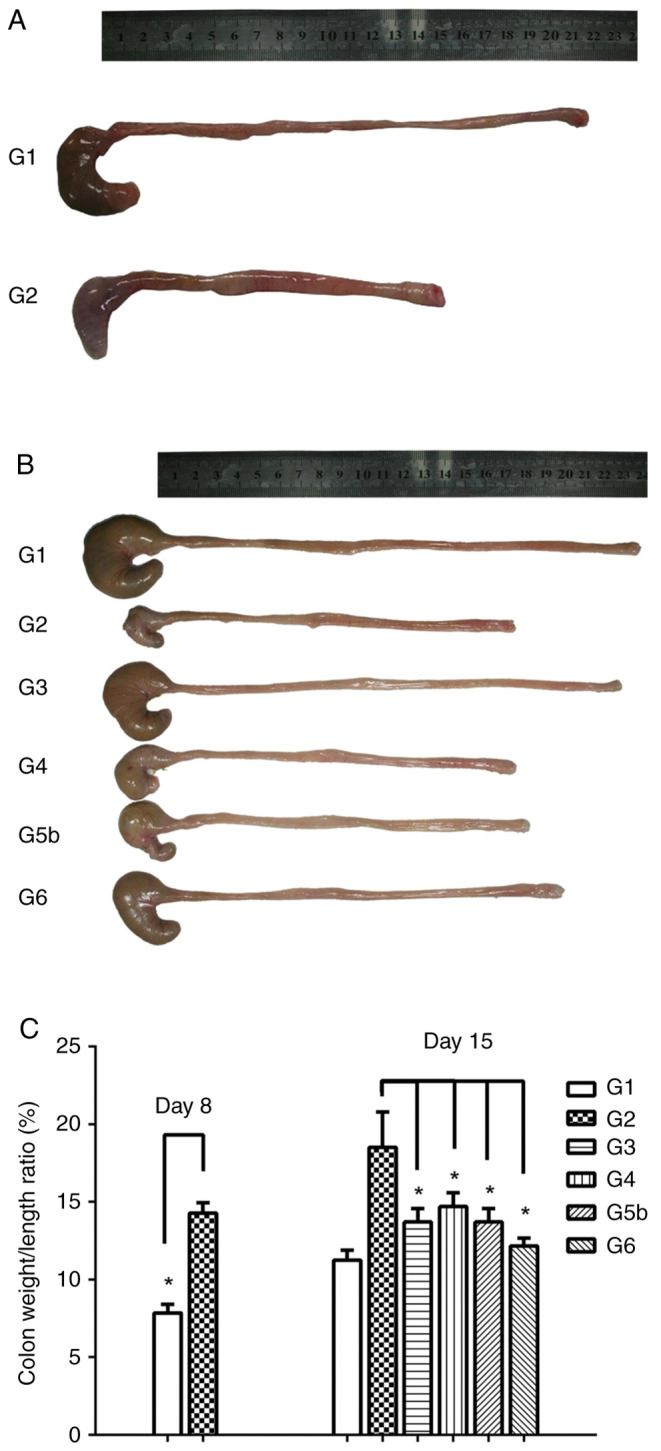
Vitamin D and vitamin E inhibit DSS-induced colonic inflammation. (A) Macroscopic inflammation was assessed, and the length of colons was measured for rats in G1 and G2 on day 8. Colon length was shorter in G2 than that in G1. (B) Colon length was measured for rats in the six groups on day 15. Compared with that in G2, the colon length of rats in G3 and G6 was markedly longer. (C) Colon weight to length ratio (%) in G1 and G2 on day 8, and in G1, G2, G3, G4, G5 and G6 on day 15. Compared with that in G2, the colon weight/length ratio in G3, G4, G5b and G6 was significantly decreased. Data are presented as the mean ± SEM. n=6. *P<0.05. G1, control group; G2, 5% DSS group; G3, prednisolone group; G4, vitamin D group; G5a-c, vitamin E (low, medium and high) groups; G6, vitamin D + vitamin E group; DSS, dextran sodium sulfate.
In addition, the colon weight/length ratio (%) in G1 and G2 on day 8 was calculated. Compared with that in G1, the colon weight/length ratio on day 8 in G2 was significantly increased (F=-5.38, P=0.01), which suggested that the rat model of DSS-induced UC was successfully established (Fig. 4C). Compared with that in G2, the colon weight/length ratio on day 15 in G3, G4, G5b and G6 was significantly lower (F3=5.67, P=0.03; F4=3.83, P=0.049; F5b=4.08, P=0.048; F6=4.97, P=0.04), indicating that both vitamin D and vitamin E inhibited DSS-induced colonic inflammation.
Vitamin D and vitamin E promote the recovery of DSS-induced UC in rats
One-half of the rats in G1 and G2 were sacrificed after anesthesia, and their colon tissues were collected for histopathological examination on day 8. Compared with in G1 (Fig. 5A), rats in G2 exhibited edema, extensive ulceration of the epithelial layer, crypt damage to the bowel wall, and infiltration of granulocytes and mononuclear cells into the mucosa (Fig. 5B), which revealed that DSS successfully induced UC in the rat model.
Figure 5.
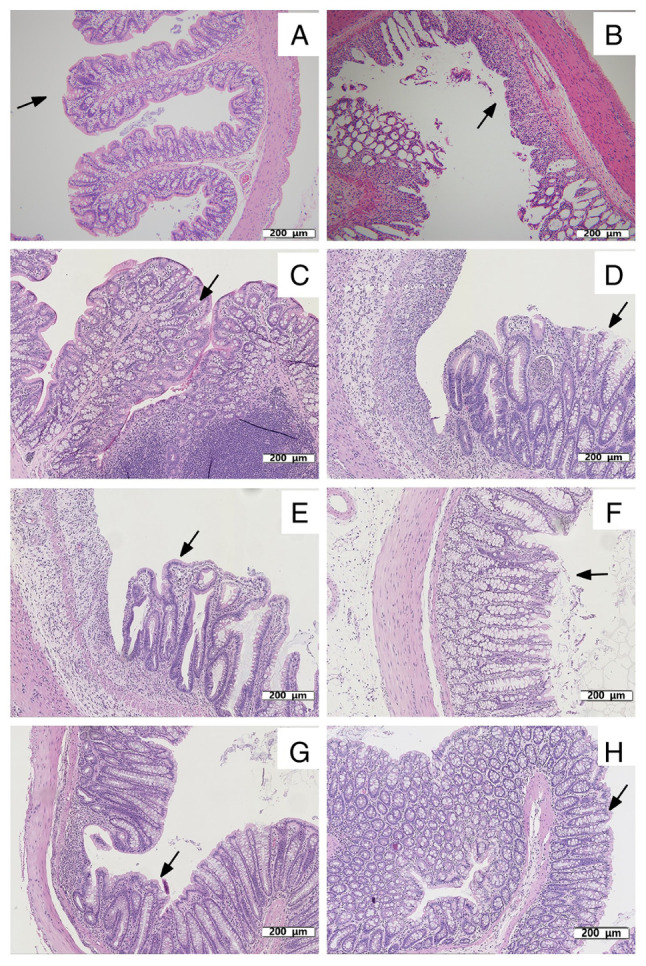
Histopathological examination of colon tissues of rats. (A) Colon tissue of rats in G1 on day 8. The mucosa is intact and free of inflammatory cell infiltration, as indicated by the arrow. (B) Acute colitis induced by DSS on day 8. Mucosal injury was shown as focal ulceration, epithelial necrosis and infiltration of inflammatory cells, as indicated by the arrow. Colon tissue of rats in (C) G1, (D) G2, (E) G3, (F) G4 (G) G5b and (H) G6 on day 15. (C) Colon tissue of rats in G1 on day 15. Intact mucosa and no inflammatory cell infiltration was indicated by arrows. (D) Colon tissue of rats in G2 on day 15. The mucosal injury was characterized by focal ulceration, epithelial necrosis and infiltration of inflammatory cells, as indicated by arrows. (E) Colon tissue of rats in G3 treated with prednisolone on day 15. Colon tissue exhibited a relatively intact mucosa with only a small infiltration of inflammatory cells, as indicated by arrows. (F) Colon tissue of rats in G4 treated with vitamin D on day 15. The colonic mucosa was intact but with some epithelial cell necrosis and infiltration of inflammatory cells, as indicated by the arrows. (G) Colon tissue of rats in G5b treated with vitamin E on day 15. The colonic mucosa was intact and infiltrated by a few inflammatory cells, as indicated by the arrows. (H) Colon tissue of rats in G6 treated with vitamin D and vitamin E on day 15. The colonic tissue mucosa was more intact than that in G4 and G5b, as indicated by the arrows. Treatment with vitamin D, vitamin E, vitamin D + vitamin E and prednisolone reduced the morphological alterations associated with DSS administration and protected the mucosal architecture. Colon tissues in the figures were all stained with hematoxylin and eosin. Scale bar, 200 µm; magnification, x110. G1, control group; G2, 5% DSS group; G3, prednisolone group; G4, vitamin D group; G5a-c, vitamin E (low, medium and high) groups; G6, vitamin D + vitamin E group; DSS, dextran sodium sulfate.
On day 15, the remaining rats were sacrificed after anesthesia, and their colon tissues were also collected for histopathological examination and detection of cytokines. The colon tissues from rats in G1 did not exhibit inflammation (Fig. 5C). Compared with rats in G2 (Fig. 5D), treatment with prednisolone (Fig. 5E), vitamin D (Fig. 5F), vitamin E (medium) (Fig. 5G), and vitamin D + vitamin E (Fig. 5H) reduced DSS-induced UC, including the severity of inflammation, extent of injury and crypt damage. The histopathological examinations of colon tissues suggested that vitamin D, vitamin E and their combination ameliorated DSS-induced UC and promoted recovery.
Vitamin D and vitamin E decrease the levels of inflammatory mediators
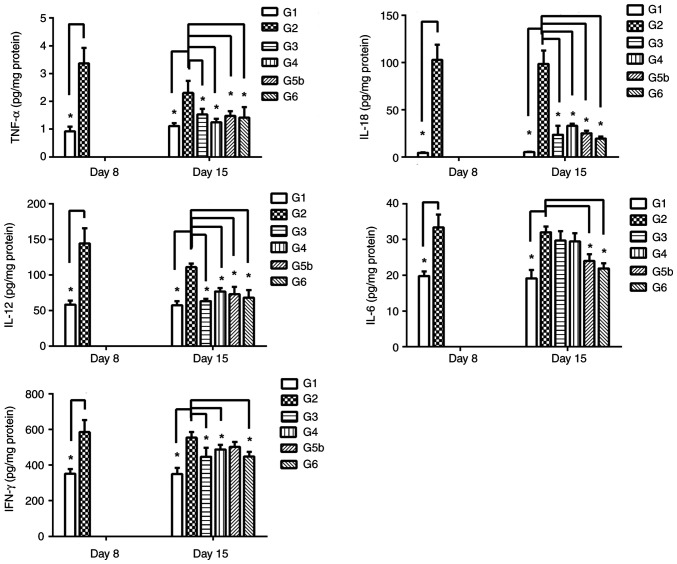
To determine the anti-inflammatory effects of vitamin D and vitamin E on the rat model of DSS-induced UC, five inflammatory markers (IL-6, IL-12, IL-18, TNF-α and IFN-γ) were assessed. Compared with those in G1, the levels of the five cytokines in G2 were significantly elevated on day 8 (FTNF-α=-2.99, P=0.03; FIFN-γ=-172.53, P=0.04; FIL-18=16.39, P=0.01; FIL-12=33.76, P=0.03; FIL-6=2.88, P=0.04; Fig. 6).
Figure 6.
Effects of prednisolone, and vitamin D and vitamin E, alone and combined, on the levels of IL-6, IL-12, IL-18, TNF-α and IFN-γ in colon tissues at various time points. The levels of these five cytokines were analyzed on day 8 in colon tissues from G1 and G2. The levels of the five cytokines in G2 were significantly elevated compared with those in G1. On day 15, the levels of the five cytokines were analyzed in G1, G2, G3, G4, G5b and G6. Data are presented as the mean ± SEM. n=6. *P<0.05. G1, control group; G2, 5% DSS group; G3, prednisolone group; G4, vitamin D group; G5a-c, vitamin E (low, medium and high) groups; G6, vitamin D + vitamin E group; DSS, dextran sodium sulfate; IFN-γ, interferon-γ; IL, interleukin; TNF-α, tumor necrosis factor-α.
On day 15, the remaining rats were sacrificed, and their colon tissues were collected for the analysis of cytokines. The results showed that the levels of four of the inflammatory cytokines (TNF-α, IL-18, IL-12 and IFN-γ) in both G3 and G4 were significantly decreased compared with those in G2 (TNF-α: G3 vs. G2 group: F=-3.82, P=0.01; G4 vs. G2 group, F=-8.81, P=0.01. IFN-γ:G3 vs. G2 group: F=138.62, P=0.01; G4 vs. G2 group, F=122.99, P=0.02. IL-18:G3 vs. G2 group: F=51.04, P=0.01; G4 vs. G2 group, F=22.17, P=0.01. IL-12: G3 vs. G2 group: F=42.09, P=0.01; G4 vs. G2 group, F=13.78, P=0.04.), whereas there was no obvious change in IL-6 levels (G3 vs. G2 group: F=0.66, P=0.65; G4 vs. G2 group, F=0.49, P=0.81) (Fig. 6). In addition, in the colon tissues, the levels of four of the inflammatory cytokines (TNF-α, IL-18, IL-12 and IL-6) were decreased in G5b compared with in G2 (FIL-12=-34.97, P=0.03; FIFN-γ=2.86, P=0.049; FTNF-α=-3.91, P=0.03; FIL-6=-8.54, P=0.04; FIL-18=46.19, P=0.01), whereas there was no obvious change in the IFN-γ levels (F=1.75, P=0.14) (Fig. 6). Notably, the levels of five of the inflammatory cytokines (IL-6, IL-12, IL-18, TNF-α and IFN-γ) were significantly reduced in G6 compared with those in G2 (FTNF-α=-4.77, P=0.03; FIFN-γ=138.66, P=0.04; FIL-12=42.18, P=0.03; FIL-6=8.04, P=0.01; FIL-18=17.39, P=0.01; Fig. 6). Collectively, these data showed that both vitamin D and vitamin E could partly reduce the levels of inflammatory cytokines in rats with DSS-induced UC, whereas their combined application exhibited more noticeable inhibitory effects.
Discussion
IBD is commonly associated with immune dysregulation; however, the precise physiological mechanisms underlying this pathological state require further investigation, so that therapeutic countermeasures can be improved. Prednisolone is currently a commonly used drug for the treatment of severe cases of IBD; therefore, in the present study, vitamins E and D were compared with it to explore whether these vitamins are more effective and thus provide a better treatment option. In the present study, CO scoring, histopathological examination and ELISA were performed on a rat model of DSS-induced UC; the results revealed that both vitamin E and vitamin D positively promoted recovery from DSS-induced UC, although their effects on the regulation of inflammatory cytokines may differ. Hahn et al (33) noted that the combination of omega-3 fatty acids and vitamin D was more beneficial for decreasing the risk of autoimmune diseases, whereas further research is required to determine whether this combination is advantageous for attenuating the incidence of IBD. The present study also indicated that the combined use of vitamin E and vitamin D may be associated with more noticeable anti-inflammatory effects on UC than their separate application.
In the majority of patients with IBD, immune pathogenesis is associated with the increased production of proinflammatory cytokines, such as IL-6, IL-12, IL-18, TNF-α and IFN-γ. Drugs targeting these cytokines or antibodies have exhibited therapeutic effects on IBD (7,12,55); thus, these cytokines are widely accepted as markers of UC. DSS-induced inflammation has previously been shown to be associated with the elevated production of IL-6, IL-18 and IFN-γ, and it could be attenuated in the absence of IL-6, IL-18 or IFN-γ (56). Therefore, this model is widely accepted for the analysis of the influence of any given drug or compound on the promotion of epithelial cell repair and attenuation of production of inflammatory mediators in animals with UC (50,57). Studies have shown that even though the absorption capacity and benefits of vitamin D vary by age and category, optimal maintenance concentrations can reduce the risk of inflammation and autoimmune diseases, as well as partly providing innate autoimmunity, especially for COVID-19 (34,36).
From the perspective of clinical symptoms and data from autopsies, the administration of DSS to rats in drinking water resulted in BW loss, shortening of the colon, mucosal inflammation and epithelial damage, which indicated that this rat model of IBD may be a good model for studying and evaluating human colitis.
Vitamin E (30 and 150 IU/kg), vitamin D (50 ng), and their combination prevented BW loss, relieved the symptoms of UC and promoted the recovery of DSS-induced UC in the present study. These data strongly indicated that both vitamin E and vitamin D had obvious anti-inflammatory effects on UC and may have positive therapeutic effects on patients with IBD. As vitamin E and vitamin D are relatively safe drugs that have long been used clinically, they could be appropriate for the treatment of IBD. In addition, vitamin D has been shown to prevent osteoporosis in the elderly, while it increases resistance to COVID-19 in appropriate concentrations (35). In women and children, vitamin D deficiency can lead to placental dysgenesis, affecting the innate development and immunity of infants and children (32,35,37).
Notably, as an antioxidant, the anti-inflammatory mechanism of vitamin E (30 IU/kg) may differ slightly from that of prednisolone (1 mg/kg) and vitamin D (50 ng), as indicated by the different levels of inflammatory cytokines detected in the present study. In addition, vitamin E plays an important role in immunity. According to recent studies, vitamin E, which has been detected in higher concentrations in immune cells compared with other blood cells, affects the development and functional regulation of DCs, macrophages, natural killer cells, T cells and B cells (58,59). According to the results of the present study, vitamin E administration (30 IU/kg) reduced the levels of TNF-α, IL-12, IL-18 and IL-6 compared with those in the DSS group, whereas it had no significant effect on IFN-γ levels. By contrast, prednisolone and vitamin D decreased the levels of TNF-α, IL-12, IL-18 and IFN-γ, but not IL-6. Acute inflammation in DSS-induced UC could be predominantly activated through the T helper (Th)2-mediated inflammatory response in the chronic state (lower levels of TNF-α, and elevated levels of IL-6, IFN-γ, IL-4 and IL-10) (50,57,60,61). Thus, the anti-inflammatory effects of vitamin E may mainly target Th2 cytokines, enabling us to find new drugs based on the Th2 cytokine mechanism, as the side effects of vitamin E are more tolerable than those of prednisolone. However, compared with that in the DSS group in the present study, colon length in the vitamin D- and vitamin E-treated groups was not significantly increased; however, there were some significant differences between these groups regarding colon weight/length ratio and inflammatory cytokine levels, especially TNF-α, IL-18 and IL-12, which may indicate that colon length is not a very sensitive indicator compared with cytokine levels. Therefore, although vitamin D and vitamin E are effective, they are not as significant in changing colon length as their combination.
In the present study, the rat model of DSS-induced UC could not thoroughly reflect the symptoms of patients with IBD. Thus, further studies are required to examine the effects of vitamin E, vitamin D and their combination on UC.
As the rat model of DSS-induced UC could not fully represent the complexity of human models of IBD, additional research on the protective and therapeutic effects of vitamin E and vitamin D for human UC should be performed. Additionally, in order to provide more valuable information for combination therapy, further studies need to be conducted to evaluate their effects in combination with other anti-inflammatory drugs. In conclusion, the combination of vitamin E and vitamin D proved to be feasible and effective in the present study. This suggests that the addition of vitamin E and vitamin D oral therapy may be an effective treatment for IBD in the future.
Acknowledgements
The authors would like to thank Dr Trevor Smith (Wolfson Centre for Age-Related Diseases, King's College London) for his technical assistance and editing of the manuscript.
Funding Statement
Funding: This study was financially supported by the National Key Technologies R&D Program for New Drugs (grant nos. 2013ZX09302303 and 2012ZX09301003-001-008), the National Natural Science Foundation of China (grant no. 82073833), the Beijing Municipal Natural Science Foundation (grant nos. 7142123 and Z131100006513010) and the State Key Laboratory of Proteomics Foundation (grant no. SKLP-YB201403).
Availability of data and material
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
XF performed multiple experiments, data acquisition and data analysis. JieY collected experimental data and participated in revising the manuscript. JiyeY contributed to the experimental design and participated in completing the relevant experiments. XW and RD contributed to the design of the study, wrote the original manuscript, confirmed the authenticity of all raw data, and agreed to be accountable for all aspects of the study in ensuring that questions related to the accuracy or integrity of any part of the study are appropriately investigated and resolved. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
Experiments were performed in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (62). The experimental procedures were approved by the National Beijing Center for Drug Safety Evaluation and Research Laboratory Animal Welfare Ethics Committee (Beijing, China; approval no. IACUC-2011-002).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Rufo PA, Denson LA, Sylvester FA, Szigethy E, Sathya P, Lu Y, Wahbeh GT, Sena LM, Faubion WA. Health supervision in the management of children and adolescents with IBD: NASPGHAN recommendations. J Pediatr Gastroenterol Nutr. 2012;55:93–108. doi: 10.1097/MPG.0b013e31825959b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lucendo AJ, Hervías D, Roncero Ó, Lorente R, Bouhmidi A, Angueira T, Verdejo C, Salueña I, González-Castillo S, Arias Á. Epidemiology and temporal trends (2000-2012) of inflammatory bowel disease in adult patients in a central region of Spain. Eur J Gastroenterol Hepatol. 2014;26:1399–1407. doi: 10.1097/MEG.0000000000000226. [DOI] [PubMed] [Google Scholar]
- 3.Rescigno M. The pathogenic role of intestinal flora in IBD and colon cancer. Curr Drug Targets. 2008;9:395–403. doi: 10.2174/138945008784221125. [DOI] [PubMed] [Google Scholar]
- 4.Ng SC, Tang W, Ching JY, Wong M, Chow CM, Hui AJ, Wong TC, Leung VK, Tsang SW, Yu HH, et al. Incidence and phenotype of inflammatory bowel disease based on results from the Asia-pacific Crohn's and colitis epidemiology study. Gastroenterology. 2013;145:158–165.e2. doi: 10.1053/j.gastro.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 5.Kuenzig ME, Fung SG, Marderfeld L, Mak JWY, Kaplan GG, Ng SC, Wilson DC, Cameron F, Henderson P, Kotze PG, et al. Twenty-first century trends in the global epidemiology of pediatric-onset inflammatory bowel disease: Systematic review. Gastroenterology. 2022;162:1147–1159.e4. doi: 10.1053/j.gastro.2021.12.282. [DOI] [PubMed] [Google Scholar]
- 6.Bauer C, Duewell P, Mayer C, Lehr HA, Fitzgerald KA, Dauer M, Tschopp J, Endres S, Latz E, Schnurr M. Colitis induced in mice with dextran sulfate sodium (DSS) is mediated by the NLRP3 inflammasome. Gut. 2010;59:1192–1199. doi: 10.1136/gut.2009.197822. [DOI] [PubMed] [Google Scholar]
- 7.Kojouharoff G, Hans W, Obermeier F, Männel DN, Andus T, Schölmerich J, Gross V, Falk W. Neutralization of tumour necrosis factor (TNF) but not of IL-1 reduces inflammation in chronic dextran sulphate sodium-induced colitis in mice. Clin Exp Immunol. 1997;107:353–358. doi: 10.1111/j.1365-2249.1997.291-ce1184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsunaga H, Hokari R, Ueda T, Kurihara C, Hozumi H, Higashiyama M, Okada Y, Watanabe C, Komoto S, Nakamura M, et al. Physiological stress exacerbates murine colitis by enhancing proinflammatory cytokine expression that is dependent on IL-18. Am J Physiol Gastrointest Liver Physiol. 2011;301:G555–G564. doi: 10.1152/ajpgi.00482.2010. [DOI] [PubMed] [Google Scholar]
- 9.Takagi H, Kanai T, Okazawa A, Kishi Y, Sato T, Takaishi H, Inoue N, Ogata H, Iwao Y, Hoshino K, et al. Contrasting action of IL-12 and IL-18 in the development of dextran sodium sulphate colitis in mice. Scand J Gastroenterol. 2003;38:837–844. doi: 10.1080/00365520310004047. [DOI] [PubMed] [Google Scholar]
- 10.Hans W, Schölmerich J, Gross V, Falk W. Interleukin-12 induced interferon-gamma increases inflammation in acute dextran sulfate sodium induced colitis in mice. Eur Cytokine Netw. 2000;11:67–74. [PubMed] [Google Scholar]
- 11.Wang Y, Tong J, Chang B, Wang BF, Zhang D, Wang BY. Genetic polymorphisms in the IL-18 gene and ulcerative colitis risk: A meta-analysis. DNA Cell Biol. 2014;33:438–447. doi: 10.1089/dna.2013.2310. [DOI] [PubMed] [Google Scholar]
- 12.Zhu Y, Mahon BD, Froicu M, Cantorna MT. Calcium and 1 alpha,25-dihydroxyvitamin D3 target the TNF-alpha pathway to suppress experimental inflammatory bowel disease. Eur J Immunol. 2005;35:217–224. doi: 10.1002/eji.200425491. [DOI] [PubMed] [Google Scholar]
- 13.Stio M, Martinesi M, Bruni S, Treves C, d'Albasio G, Bagnoli S, Bonanomi AG. Interaction among vitamin D(3) analogue KH 1060, TNF-alpha, and vitamin D receptor protein in peripheral blood mononuclear cells of inflammatory bowel disease patients. Int Immunopharmacol. 2006;6:1083–1092. doi: 10.1016/j.intimp.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 14.Magro F, Cordeiro G, Dias AM, Estevinho MM. Inflammatory bowel disease-non-biological treatment. Pharmacol Res. 2020;160(105075) doi: 10.1016/j.phrs.2020.105075. [DOI] [PubMed] [Google Scholar]
- 15.Jeong DY, Kim S, Son MJ, Son CY, Kim JY, Kronbichler A, Lee KH, Shin JI. Induction and maintenance treatment of inflammatory bowel disease: A comprehensive review. Autoimmun Rev. 2019;18:439–454. doi: 10.1016/j.autrev.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Jiang Y, Xia X, Wang W, Lin L, Xu C, Cai Z, Zheng B, Pei J, Shen S, Xia B. Hyperhomocysteinemia and related genetic polymorphisms correlate with ulcerative colitis in Chinese Han population in Central China [corrected] Cell Biochem Biophys. 2012;62:203–210. doi: 10.1007/s12013-011-9283-4. [DOI] [PubMed] [Google Scholar]
- 17.Hart AL. Vitamin D and inflammatory bowel disease: Chicken or egg? Inflamm Bowel Dis. 2013;19:459–460. doi: 10.1002/ibd.23031. [DOI] [PubMed] [Google Scholar]
- 18.Levin AD, Wadhera V, Leach ST, Woodhead HJ, Lemberg DA, Mendoza-Cruz AC, Day AS. Vitamin D deficiency in children with inflammatory bowel disease. Dig Dis Sci. 2011;56:830–836. doi: 10.1007/s10620-010-1544-3. [DOI] [PubMed] [Google Scholar]
- 19.Blanck S, Aberra F. Vitamin d deficiency is associated with ulcerative colitis disease activity. Dig Dis Sci. 2013;58:1698–1702. doi: 10.1007/s10620-012-2531-7. [DOI] [PubMed] [Google Scholar]
- 20.Hassan V, Hassan S, Seyed-Javad P, Ahmad K, Asieh H, Maryam S, Farid F, Siavash A. Association between serum 25 (OH) vitamin D concentrations and inflammatory bowel diseases (IBDs) activity. Med J Malaysia. 2013;68:34–38. [PubMed] [Google Scholar]
- 21.Selhub J, Byun A, Liu Z, Mason JB, Bronson RT, Crott JW. Dietary vitamin B6 intake modulates colonic inflammation in the IL10-/- model of inflammatory bowel disease. J Nutr Biochem. 2013;24:2138–2143. doi: 10.1016/j.jnutbio.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li YC. Investigating the role of vitamin D in IBD pathophysiology and treatment. Gastroenterol Hepatol (N Y) 2008;4:20–21. [PMC free article] [PubMed] [Google Scholar]
- 23.Jankowska M, Trzonkowski P, Dębska-Ślizień A, Marszałł M, Rutkowski B. Vitamin B6 status, immune response and inflammation markers in kidney transplant recipients treated with polyclonal anti-thymocyte globulin. Transplant Proc. 2014;46:2631–2635. doi: 10.1016/j.transproceed.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 24.De Silva P, Ananthakrishnan AN. Vitamin D and IBD: More pieces to the puzzle, still no complete picture. Inflamm Bowel Dis. 2012;18:1391–1393. doi: 10.1002/ibd.22854. [DOI] [PubMed] [Google Scholar]
- 25.Xue LN, Xu KQ, Zhang W, Wang Q, Wu J, Wang XY. Associations between vitamin D receptor polymorphisms and susceptibility to ulcerative colitis and Crohn's disease: A meta-analysis. Inflamm Bowel Dis. 2013;19:54–60. doi: 10.1002/ibd.22966. [DOI] [PubMed] [Google Scholar]
- 26.Zator ZA, Cantu SM, Konijeti GG, Nguyen DD, Sauk J, Yajnik V, Ananthakrishnan AN. Pretreatment 25-hydroxyvitamin D levels and durability of anti-tumor necrosis factor-α therapy in inflammatory bowel diseases. JPEN J Parenter Enteral Nutr. 2014;38:385–391. doi: 10.1177/0148607113504002. [DOI] [PubMed] [Google Scholar]
- 27.Ooi JH, Li Y, Rogers CJ, Cantorna MT. Vitamin D regulates the gut microbiome and protects mice from dextran sodium sulfate-induced colitis. J Nutr. 2013;143:1679–1686. doi: 10.3945/jn.113.180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mackawy AMH, Badawi MEH. Association of vitamin D and vitamin D receptor gene polymorphisms with chronic inflammation, insulin resistance and metabolic syndrome components in type 2 diabetic Egyptian patients. Meta Gene. 2014;2:540–556. doi: 10.1016/j.mgene.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li YC, Chen Y, Du J. Critical roles of intestinal epithelial vitamin D receptor signaling in controlling gut mucosal inflammation. J Steroid Biochem Mol Biol. 2015;148:179–183. doi: 10.1016/j.jsbmb.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leslie WD, Miller N, Rogala L, Bernstein CN. Vitamin D status and bone density in recently diagnosed inflammatory bowel disease: The manitoba IBD cohort study. Am J Gastroenterol. 2008;103:1451–1459. doi: 10.1111/j.1572-0241.2007.01753.x. [DOI] [PubMed] [Google Scholar]
- 31.Ghaly S, Lawrance I. The role of vitamin D in gastrointestinal inflammation. Expert Rev Gastroenterol Hepatol. 2014;8:909–923. doi: 10.1586/17474124.2014.925796. [DOI] [PubMed] [Google Scholar]
- 32.Phillips EA, Hendricks N, Bucher M, Maloyan A. Vitamin D supplementation improves mitochondrial function and reduces inflammation in placentae of obese women. Front Endocrinol (Lausanne) 2022;13(893848) doi: 10.3389/fendo.2022.893848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hahn J, Cook NR, Alexander EK, Friedman S, Walter J, Bubes V, Kotler G, Lee IM, Manson JE, Costenbader KH. Vitamin D and marine omega 3 fatty acid supplementation and incident autoimmune disease: VITAL randomized controlled trial. BMJ. 2022;376(e066452) doi: 10.1136/bmj-2021-066452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bui L, Zhu Z, Hawkins S, Cortez-Resendiz A, Bellon A. Vitamin D regulation of the immune system and its implications for COVID-19: A mini review. SAGE Open Med. 2021;9(20503121211014073) doi: 10.1177/20503121211014073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hribar CA, Cobbold PH, Church FC. Potential role of vitamin D in the elderly to resist COVID-19 and to slow progression of Parkinson's disease. Brain Sci. 2020;10(284) doi: 10.3390/brainsci10050284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Charoenngam N, Holick MF. Immunologic effects of vitamin D on human health and disease. Nutrients. 2020;12(2097) doi: 10.3390/nu12072097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mailhot G, White JH. Vitamin D and immunity in infants and children. Nutrients. 2020;12(1233) doi: 10.3390/nu12051233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao H, Zhang H, Wu H, Li H, Liu L, Guo J, Li C, Shih DQ, Zhang X. Protective role of 1,25(OH)2 vitamin D3 in the mucosal injury and epithelial barrier disruption in DSS-induced acute colitis in mice. BMC Gastroenterol. 2012;12(57) doi: 10.1186/1471-230X-12-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vaghari-Tabari M, Mohammadzadeh I, Qujeq D, Majidinia M, Alemi F, Younesi S, Mahmoodpoor A, Maleki M, Yousefi B, Asemi Z. Vitamin D in respiratory viral infections: A key immune modulator? Crit Rev Food Sci Nutr. 2021:1–16. doi: 10.1080/10408398.2021.1972407. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 40.Colón AL, Madrigal JL, Menchén LA, Moro MA, Lizasoain I, Lorenzo P, Leza JC. Stress increases susceptibility to oxidative/nitrosative mucosal damage in an experimental model of colitis in rats. Dig Dis Sci. 2004;49:1713–1721. doi: 10.1023/b:ddas.0000043391.64073.e4. [DOI] [PubMed] [Google Scholar]
- 41.Narushima S, Spitz DR, Oberley LW, Toyokuni S, Miyata T, Gunnett CA, Buettner GR, Zhang J, Ismail H, Lynch RG, Berg DJ. Evidence for oxidative stress in NSAID-induced colitis in IL10-/- mice. Free Radic Biol Med. 2003;34:1153–1166. doi: 10.1016/s0891-5849(03)00065-0. [DOI] [PubMed] [Google Scholar]
- 42.Seven A, Seymen O, Inci F, Oz B, Yiğit G, Burçak G. Evaluation of oxidative stress in experimental colitis: Effects of L-arginine-nitric oxide pathway manipulation. J Toxicol Environ Health A. 2000;61:167–176. doi: 10.1080/00984100050131314. [DOI] [PubMed] [Google Scholar]
- 43.Rana SV, Sharma S, Kaur J, Prasad KK, Sinha SK, Kochhar R, Malik A, Morya RK. Relationship of cytokines, oxidative stress and GI motility with bacterial overgrowth in ulcerative colitis patients. J Crohns Colitis. 2014;8:859–865. doi: 10.1016/j.crohns.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 44.Rana SV, Sharma S, Prasad KK, Sinha SK, Singh K. Role of oxidative stress & antioxidant defence in ulcerative colitis patients from north India. Indian J Med Res. 2014;139:568–571. [PMC free article] [PubMed] [Google Scholar]
- 45.Korkina L, Suprun M, Petrova A, Mikhal'chik E, Luci A, De Luca C. The protective and healing effects of a natural antioxidant formulation based on ubiquinol and Aloe vera against dextran sulfate-induced ulcerative colitis in rats. Biofactors. 2003;18:255–264. doi: 10.1002/biof.5520180228. [DOI] [PubMed] [Google Scholar]
- 46.Abdala-Valencia H, Berdnikovs S, Cook-Mills JM. Vitamin E isoforms as modulators of lung inflammation. Nutrients. 2013;5:4347–4363. doi: 10.3390/nu5114347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Almohanna HM, Ahmed AA, Tsatalis JP, Tosti A. The role of vitamins and minerals in hair loss: A review. Dermatol Ther (Heidelb) 2019;9:51–70. doi: 10.1007/s13555-018-0278-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carrier J, Aghdassi E, Cullen J, Allard JP. Iron supplementation increases disease activity and vitamin E ameliorates the effect in rats with dextran sulfate sodium-induced colitis. J Nutr. 2002;132:3146–3150. doi: 10.1093/jn/131.10.3146. [DOI] [PubMed] [Google Scholar]
- 49.Tahan G, Aytac E, Aytekin H, Gunduz F, Dogusoy G, Aydin S, Tahan V, Uzun H. Vitamin E has a dual effect of anti-inflammatory and antioxidant activities in acetic acid-induced ulcerative colitis in rats. Can J Surg. 2011;54:333–338. doi: 10.1503/cjs.013610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parmar AR, Trivedi PP, Jena GB. Dextran sulfate sodium-induced ulcerative colitis leads to testicular toxicity in mice: Role of inflammation, oxidative stress and DNA damage. Reprod Toxicol. 2014;49:171–184. doi: 10.1016/j.reprotox.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 51.Hu D, Wu CQ, Li ZJ, Liu Y, Fan X, Wang QJ, Ding RG. Characterizing the mechanism of thiazolidinedione-induced hepatotoxicity: An in vitro model in mitochondria. Toxicol Appl Pharmacol. 2015;284:134–141. doi: 10.1016/j.taap.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 52.Meng G, Zhao J, Wang HM, Ding RG, Zhang XC, Huang CQ, Ruan JX. Injury of cell tight junctions and changes of actin level in acute lung injury caused by the perfluoroisobutylene exposure and the role of Myosin light chain kinase. J Occup Health. 2011;53:250–257. doi: 10.1539/joh.10-0055-oa. [DOI] [PubMed] [Google Scholar]
- 53.Weng XC, Fan X, Wang QX, Shi C, Li LN, Ouyang ZH, Kong Q, Wang QJ, Guan YB, Ding RG. Research on the methods of active systemic anaphylaxis on guinea pig in drug safety evaluation. Chin J Comp Med. 2012;22:51–55. [Google Scholar]
- 54.Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238–249. [PubMed] [Google Scholar]
- 55.Ten Hove T, Corbaz A, Amitai H, Aloni S, Belzer I, Graber P, Drillenburg P, van Deventer SJ, Chvatchko Y, Te Velde AA. Blockade of endogenous IL-18 ameliorates TNBS-induced colitis by decreasing local TNF-alpha production in mice. Gastroenterology. 2001;121:1372–1379. doi: 10.1053/gast.2001.29579. [DOI] [PubMed] [Google Scholar]
- 56.Wang L, Feng Y, Wang J, Luo T, Wang X, Wu M, Wang R, Chen D, Li J, Wang J. Arbutin ameliorates murine colitis by inhibiting JAK2 signaling pathway. Front Pharmacol. 2021;12(683818) doi: 10.3389/fphar.2021.683818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chassaing B, Aitken JD, Malleshappa M, Vijay-Kumar M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr Protoc Immunol. 2014;104:15.25.1–15.25.14. doi: 10.1002/0471142735.im1525s104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sun M, Yan Z, Sun R, Tian W, Yi W, Zhang J. Dynamic monitoring and a clinical correlation analysis of the serum vitamin A, D, and E levels in children with recurrent respiratory tract infections. Am J Transl Res. 2022;14:3533–3538. [PMC free article] [PubMed] [Google Scholar]
- 59.Lewis ED, Meydani SN, Wu D. Regulatory role of vitamin E in the immune system and inflammation. IUBMB Life. 2019;71:487–494. doi: 10.1002/iub.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Perše M, Cerar A. Dextran sodium sulphate colitis mouse model: Traps and tricks. J Biomed Biotechnol. 2012;2012(718617) doi: 10.1155/2012/718617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schwager J, Seifert N, Bompard A, Raederstorff D, Bendik I. Resveratrol, EGCG and vitamins modulate activated T lymphocytes. Molecules. 2021;26(5600) doi: 10.3390/molecules26185600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals: The national academies collection: Reports funded by national institutes of health. In: Guide for the care and use of laboratory animals. National Academies Press (US) Copyright ©. 2011, National Academy of Sciences, Washington (DC), 2011. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.