Abstract
Background:
Up to 10% of patients with advanced non-small cell lung cancer (aNSCLC) have pre-existing interstitial lung disease (ILD). These patients are usually excluded from immunotherapy clinical trials. Consequently, knowledge on outcomes following nivolumab treatment in these patients remains limited. The primary objective of this study was to evaluate survival outcome following nivolumab treatment in ILD patients with pre-treated aNSCLC in the real-world setting.
Patients and methods:
The study included all patients with aNSCLC recorded in the French hospital database, starting nivolumab in 2015–2016. Patients were stratified by pre-existing ILD and three subgroups were studied [auto-immune or granulomatous (AI/G) ILD, other known causes ILD and idiopathic ILD]. Time to discontinuation of nivolumab treatment [time to treatment duration (TTD)] and overall survival (OS) were estimated using Kaplan–Meier survival analysis.
Results:
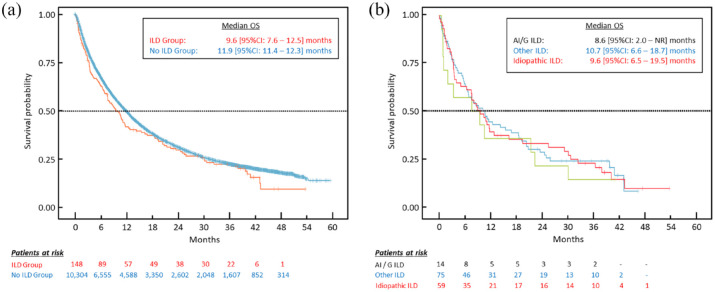
Of 10,452 aNSCLC patients initiating nivolumab, 148 (1.4%) had pre-existing ILD. Mean age at nivolumab initiation was 64.6 ± 9.4 years in ILD and 63.8 ± 9.6 years in non-ILD. Compared to non-ILD, patients in the ILD group were more frequently men (p < 0.05) and had more comorbidities (p < 0.001). There was no significant difference between ILD and non-ILD groups for median TTD (2.5 versus 2.8 months; p = 0.6) or median OS (9.6 versus 11.9 months; p = 0.1). Median OS in AI/G ILD (n = 14), other known causes ILD (n = 75), and idiopathic ILD (n = 59) were 8.6, 10.7, and 9.6 months, respectively.
Conclusion:
In this large cohort of aNSCLC patients with ILD, outcomes are similar to those obtained in the non-ILD population. Immunotherapy could be beneficial for these patients.
Keywords: immunotherapy, interstitial lung disease, nivolumab, non-small cell lung cancer, SNDS, survival
Introduction
Non-small cell lung cancer (NSCLC) is the fourth most frequent cancer in terms of incidence in France and has the highest mortality rate.1,2 The therapeutic arsenal for advanced NSCLC (aNSCLC) has expanded greatly with the development of immunotherapies. In 2015, the Checkmate-017 and -057 pivotal trials of nivolumab as a second-line therapy of aNSCLC demonstrated the efficacy of this immune checkpoint inhibitor (ICI), with significantly longer overall survival (OS) compared to docetaxel and, most notably, long-term survival of 13.4% of patients at 5 years.3–5 These findings have also been replicated in real-world studies with broader patient populations.6–9 One of these, the UNIVOC study, showed similar results for 2- and 3-year OS and response rate as the pivotal Checkmate trials.7,8
The presence of comorbid interstitial lung disease (ILD) is a complicating factor in the management of aNSCLC. In a large French study, the prevalence of all-cause ILD was 100/100,0000.10 However, in the National Lung Screening Trial performed in the United States, CT imaging revealed interstitial anomalies in 20.2% of patients with lung cancer.11 Patients with ILD have a higher risk of developing NSCLC and present a poorer prognosis and a higher rate of complications with systemic treatments, including surgery, chemotherapy, and targeted therapy.12 There is currently no standard of care for the management of patients with comorbid ILD and lung cancer. A recent international survey of 494 lung specialists by the Interstitial Lung Diseases and Thoracic Oncology assemblies of the European Respiratory Society showed that 69% of participants offered palliative care to patients with advanced ILD and Stage IV lung cancer, 31% proposed immunotherapy and 25% proposed platinum-based chemotherapy.13 Despite the relative safety of ICIs, approximately 3% of patients without ILD develop pulmonary toxicity while under immunotherapy. Patients with comorbid ILD have been excluded from most ICI trials and the incidence and prognosis of pulmonary toxicity in this population is still largely unknown, which may explain the relatively limited use of ICIs in patients with ILD. Historical cohort studies14 or prospective studies15–17 performed in Japan have reported variable rates of pulmonary toxicity (up to 30%) in ILD under treatment with nivolumab, atezolizumab, or pembrolizumab. Where efficacy data was available, a treatment response was observed.15,16 However, these findings may be difficult to extrapolate to the Caucasian population.18
Outside the Asian context, very little data is available on the utility of ICIs in patients with comorbid ILD and lung cancer. We recently reported a small case series of six patients with mild to moderate idiopathic pulmonary fibrosis (IPF) treated with nivolumab after at least one line of platinum-based chemotherapy.19 The disease control rate was 50%: one patient showed a partial response, two remained stable, and the other three progressed rapidly at the first assessment. In terms of pulmonary toxicity, two patients presented radiological and clinical signs of deterioration on treatment without acute exacerbation.
More data on the benefits and risks of nivolumab in Caucasian patients with ILD are clearly desirable. Given the large number of patients enrolled in the UNIVOC cohort (10,452), it has been possible to evaluate the relative efficacy of nivolumab in different patient subgroups, even for conditions that are uncommon. In this way, we have previously demonstrated similar efficacy of nivolumab in older patients (aged >80 years), in patients with brain metastasis and in those with chronic kidney disease.8 The aim of this study was to use the UNIVOC database to investigate whether the presence of comorbid ILD influences the efficacy of ICI in patients with aNSCLC.
Methods
This was a retrospective observational study of patients with aNSCLC and comorbid ILD in the UNIVOC cohort. This cohort represents all patients with aNSCLC starting nivolumab treatment in a second- or later-line setting in France in 2015 and 2016. Patients were identified in the French national hospital discharge database [Programme de Médicalisation des Systèmes d’Information (PMSI)]. The construction of this cohort has been described in detail previously7 and a brief summary of the key points is provided below. Patients were followed from the date of first nivolumab administration until 31 December 2019, a total potential follow-up period of 5 years.
Identification of patients and patient subgroups
The ICD-10 (International Classification of Diseases 10th Version) diagnostic codes documented on the hospital discharge summary were used to identify the patient groups of interest. The UNIVOC cohort consisted of all patients with a diagnostic code for lung cancer (C34*) associated with a documented prescription of nivolumab during the inclusion period (between 1 January 2015 and 31 December 2016). At that time, aNSCLC (squamous or non-squamous) was the only type of lung cancer for which nivolumab could be prescribed, and prescription was restricted to second- or later-line treatment. The date of first administration of nivolumab was taken as the index date for the study. Patients with ILD were identified by the diagnostic code documented on any hospital discharge summary either during the inclusion period or during the year preceding inclusion. As patients could not receive an ICI before the inclusion period, no cases of immune-related pneumonitis are presented in this cohort. Three different subgroups of patients with ILD were identified, namely auto-immune or granulomatous (AI/G) ILD, ILD due to other known causes, and idiopathic ILD (Supplemental Table 1).
Data extracted from the database
Information on selected comorbidities was extracted for all hospitalizations occurring prior to the index date using the relevant diagnostic codes on the hospital discharge summaries.
Information on administration of anticancer medication throughout the follow-up period was documented. It should be noted that individual medications are only documented by name for a restricted number of innovative expensive drugs (including nivolumab). However, all outpatient visits for chemotherapy administration are documented irrespective of whether the drug administered is explicitly named or not. No information is available on anticancer drugs delivered outside the hospital in community pharmacies.
In-hospital deaths, which account for around 80% of deaths from NSCLC in France, were documented.
Derived variables
Since the date of diagnosis is not documented in the database, the time since diagnosis of NSCLC was determined using a proxy variable consisting of the interval between the first documented hospitalization for NSCLC and the date of the first administration of nivolumab (index date).
Discontinuation of nivolumab treatment was identified by an interval of at least 6 weeks without administration of nivolumab. Since nivolumab is administered fortnightly, the date of discontinuation was defined as the last administration date plus 14 days (or, if the patient died, the date of death). The time to treatment duration (TTD) was defined as the interval between the index date and the discontinuation date.
OS was defined as the interval between the index date and the date of in-hospital death, regardless of the cause of death, or between the index date and the last documented record of the patient in the database.
Statistical analysis
Patient subgroups were compared using the χ² test for categorical variables or the Wilcoxon test for continuous variables. Kaplan–Meier survival analysis was used to determine TTD and OS, which were compared between groups using the log rank statistic. Statistical analyses were performed using R-3.6.1 software (FreeSoftware Foundation, Boston, MA, USA).
Results
Patients
The UNIVOC cohort included a total of 10,452 patients with aNSCLC who initiated a second- or higher-line treatment with nivolumab. Of these patients, 148 (1.4%) were identified with pre-existing ILD. Patients with AI/G ILD accounted for 9.5% of these patients, ILD of other known causes for around half and idiopathic ILD for the remaining patients (Figure 1).
Figure 1.
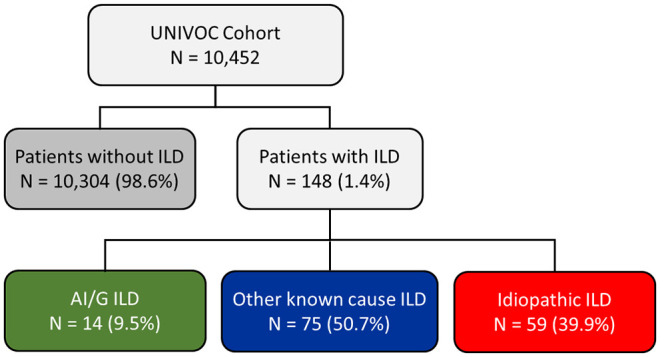
Patient selection.
AI/G, auto-immune or granulomatous; ILD, interstitial lung disease.
The characteristics of the patients in the UNIVOC cohort according to diagnostic group are presented in Table 1. The mean age at nivolumab initiation was 64.6 ± 9.4 years in the ILD group and 63.8 ± 9.6 years in patients without ILD. Compared to the latter, patients in the ILD group were more frequently men (p < 0.05), had more comorbidities (p < 0.001) and presented more frequently with squamous aNSCLC (p < 0.05). Patients with ILD also more frequently had a history of previous curative surgery or radiotherapy. The percentage of patients receiving any systemic treatment was 53.8% in the non-ILD population and 47.3% in the ILD population (p = 0.1379). The percentage of patients with systemic re-treatment was lower in patients in the AI/G subgroup (28.6%) than in the other subgroups.
Table 1.
Patient characteristics at initiation of nivolumab treatment.
| Baseline characteristics | Non-ILD population (N = 10,304) | ILD population, total (N = 148) | ILD population, AI/G subgroup (n = 14) | ILD population, ‘other’ subgroup (n = 75) | ILD population, idiopathic subgroup (n = 59) |
|---|---|---|---|---|---|
| Histology (non-squamous cell: n; %)$ | 5754 (55.8%) | 51 (34.5%) | 5 (35.7%) | 20 (26.7%) | 26 (44.1%) |
| Age (mean ± SD; years) | 63.8 ± 9.6 | 64.6 ± 9.4 | 63.4 ± 7.2 | 65.3 ± 9.4 | 64.1 ± 9.8 |
| ⩾80 years (n; %) | 506 (4.9%) | 8 (5.4%) | 0 (0%) | 5 (6.7%) | 3 (5.1%) |
| Gender (men: n, %)$ | 7303 (70.9%) | 117 (79.1%) | 9 (64.3%) | 59 (78.7%) | 49 (83.1%) |
| Time since lung cancer diagnosis (mean ± SD; months) | 19.6 ± 20.7 | 20.4 ± 19.5 | 22.5 ± 24.9 | 21.2 ± 18.3 | 19.0 ± 19.3 |
| Presence of cerebral metastases (n; %) | 1,782 (17.3%) | 18 (12.2%) | 1 (7.1%) | 11 (14.7%) | 6 (10.2%) |
| Previous curative surgery (n; %)* | 1599 (15.5%) | 30 (20.3%) | 4 (28.6%) | 15 (20%) | 11 (18.6%) |
| Previous radiotherapy (n; %)* | 2480 (24.1%) | 50 (33.8%) | 2 (14.3%) | 32 (42.7%) | 16 (27.1%) |
| Time since first chemotherapy (mean ± SD; months) | 16.4 ± 17.3 | 14.6 ± 11.9 | 11.2 ± 11.0 | 16.7 ± 12.5 | 12.8 ± 10.7 |
| Comorbidities | |||||
| Hypertension (n; %)* | 1,928 (18.7%) | 58 (39.2%) | 6 (42.9%) | 28 (37.3%) | 24 (40.7%) |
| Diabetes (n; %)* | 895 (8.7%) | 39 (26.4%) | 5 (35.7%) | 17 (22.7%) | 17 (28.8%) |
| Renal impairment (n; %)* | 460 (4.5%) | 19 (12.8%) | 1 (7.1%) | 9 (12.0%) | 9 (15.3%) |
| Chronic obstructive pulmonary disease (n; %)* | 1,297 (12.6%) | 51 (34.5%) | 4 (28.6%) | 23 (30.7%) | 24 (40.7%) |
| Pulmonary insufficiency (n; %)* | 144 (1.4%) | 9 (6.1%) | 0 (0%) | 3 (4.0%) | 6 (10.2%) |
| Other chronic pulmonary disease (n; %)* | 862 (8.4%) | 41 (27.7%) | 2 (14.3%) | 21 (28.0%) | 18 (30.5%) |
| Connective tissue diseases (n; %)* | 68 (0.7%) | 3 (2.0%) | 2 (14.3%) | 1 (1.3%) | 0 (0%) |
| Other auto-immune disease (n; %)* | 29 (0.3%) | 1 (0.7%) | 1 (7.1%) | 0 (0%) | 0 (0%) |
| Charlson index (mean ± SD)$ | 10.4 ± 4.1 | 11.4 ± 4.1 | 12.6 ± 3.2 | 11.1 ± 4.1 | 11.4 ± 4.2 |
| Any systemic re-treatment (n, %) | 5540 (53.8%) | 70 (47.3%) | 4 (28.6%) | 38 (50.7%) | 28 (47.5%) |
A significant difference between the patients with and without ILD (p < 0.001).
A significant difference between the patients with and without ILD (p ⩽ 0.037).
AI/G, auto-immune or granulomatous; ILD, interstitial lung disease; SD, standard deviation.
Nivolumab treatment duration
The median TTD for nivolumab was 2.5 months [95% CI: 2.0–3.9] in patients with ILD and 2.8 months [2.8–2.8] in those without, with no significant difference between the two groups (Table 2). Treatment persistence rates at 1- and 2-year were, respectively, 12.8% [9.0–20.3] and 5.4% [3.2–11.5] in the ILD group and 13.4% [12.8–14.1] and 5.6% [5.2–6.1] in the non-ILD group. In the subgroup with idiopathic ILD, the median TTD was 2.9 months [1.84–4.41] while the median TTD in the other causes ILD subgroup was 2.5 months [2.04–5.69]. Compared to the other subgroups, TTD was shorter (1.5 months [0.9–12.9]) in the AI/G subgroup and the 1-year persistence rate was lower (14.3% [4.0–51.5]).
Table 2.
Time to treatment discontinuation and OS in the study population and in subgroups of interest.
| Non-ILD population (N = 10,304) | ILD population, total (N = 148) | ILD population, AI/G subgroup (n = 14) | ILD population, ‘other’ subgroup (n = 75) | ILD population, idiopathic subgroup (n = 59) | |
|---|---|---|---|---|---|
| Median TTD [95% CI] (months) | 2.8 [2.8–2.8] | 2.5 [2.0–3.9] | 1.5 [0.9–12.9] | 2.5 [2.0–5.7] | 2.9 [1.8–4.1] |
| Median OS [95% CI] (months) | 11.9 [11.4–12.3] | 9.6 [7.6–12.5] | 8.6 [2.0–NR] | 10.7 [6.7–18.7] | 9.6 [6.4–19.5] |
| Survival at 1 year | 49.7% [48.8–50.7] | 40.8% [34.1–50.5] | 35.7% [17.7–72.1] | 43.0% [34.3–57.6] | 37.3% [28.2–54.5] |
| Survival at 2 years | 31.0% [30.1–32.0] | 28.7% [22.8–38.2] | 21.4% [7.9–58.4] | 27.1% [19.8–41.4] | 31.1% [22.7–48.5] |
| Survival at 3 years | 22.3% [21.4–23.2] | 21.1% [16.0–30.5] | 14.3% [4.0–51.5] | 24.0% [15.8–36.5] | 20.5% [13.8–37.7] |
AI/G, auto-immune or granulomatous; CI, confidence interval; ILD, interstitial lung disease; OS, overall survival.
Overall survival
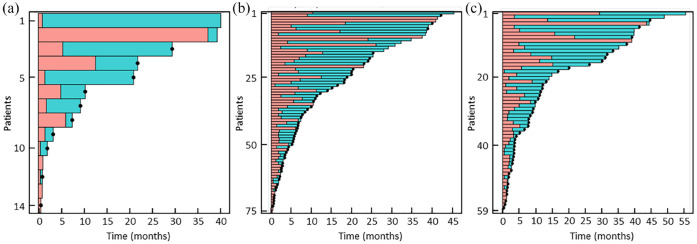
No significant difference in OS was observed between the ILD and non-ILD groups (Table 2). The corresponding Kaplan–Meier survival curves are presented in Figure 2(a). With regard to the three ILD subgroups, patient numbers were small and, consequently, the confidence intervals of the survival estimates were broad. In all subgroups, OS was broadly similar, although possibly somewhat lower in the patients with AI/G ILD. Kaplan–Meier survival curves for these subgroups are presented in Figure 2(b). Individual patient outcomes are displayed in the form of swimmer plots in Figure 3. These show that many of the included patients survive for months or years following discontinuation of nivolumab.
Figure 2.
Overall survival in ILD and non-ILD goups (a) and ILD subgroups (b). (a) Blue curve: non-ILD population; red curve: ILD population; (b) green curve: AI/G ILD subgroup; blue curve: other ILD subgroup; red curve: idiopathic ILD subgroup.
AI/G, auto-immune or granulomatous; CI, confidence interval; ILD, interstitial lung disease; OS, overall survival.
Figure 3.
Individual patient outcomes in the three ILD patients subgroups. (a) AI/G ILD. (b) Other ILD. (c) Idiopathic ILD.
AI/G, auto-immune or granulomatous; black dots, time of death; blue bars, post-treatment period; ILD, interstitial lung disease; pink bars, time treated with nivolumab.
Discussion
Our study explores OS in patients with ILD in a large French cohort treated for aNSCLC with nivolumab in the real-world setting. No significant difference in median OS between and the group without ILD was observed. However, it should be noted that patients with ILD often have other comorbidities such as diabetes and renal impairment, which have been shown to be potentially deleterious in terms of response to immunotherapy.8,20 Nevertheless, it appears that 3-year survival rates are generally similar, suggesting that when patients respond to treatment, the survival benefit is comparable. These results also highlight the clinical interest of specific treatment of cancer in patients with ongoing ILD, who achieve nearly the same OS as those without ILD. Our findings are consistent with those of a recent retrospective Japanese study by Tasaka et al.,21 who evaluated 461 patients with NSCLC, of whom 49 had ILD, initiating treatment with nivolumab or pembrolizumab in a first- or second-line setting, which reported similar OS (27.8 versus 25.2 months) and objective responses (49% versus 30.1%) for the ILD and non-ILD groups.
Although patients with concomitant cancer and ILD appear to have a higher tumor mutational burden than non-ILD patients,22 they present a comparable level of PD-L1 expression and a comparable number of intra-tumoral CD8+ lymphocytes,23,24 which are associated with improved prognosis under ICIs.25 This provides a rationale for the use of ICIs in NSCLC patients with ILD. These patients have previously been excluded from clinical trials due to a potential risk of immune-related adverse events. Nonetheless, in NSCLC in general, the occurrence of such adverse events during immunotherapy has been associated with improved outcome.26
Most of the available data on ILD, NSCLC and immunotherapy have come from Japanese studies. However, the Asian population has a genetic predisposition to developing treatment toxicity with anti-epidermal growth factor receptors (EGFRs), tyrosine kinase inhibitors (TKIs) or chemotherapy27 and are at high risk of exacerbation of their ILD, in particular for IPF.18 Although the ethnicity of the patients in the UNIVOC cohort is not documented, a recent study suggested that the Asian population accounts for less than 7% of patients diagnosed with ILD in one of the most multi-ethnic areas in France.10,28 Moreover, based on data from the national institute of statistics, non-Caucasians account for less than 5% of the total French population. Therefore, our study probably offers an insight into outcomes of ILD patients receiving ICIs for the treatment of NSCLC in a predominantly non-Asian population. This study included the largest number of patients with concurrent cancer and ILD to have been studied in Europe to date.
We were able to separate the aetiologies of ILD into three subgroups. The first group (AI/G) consisted of four patients with sarcoidosis, and 10 patients with vasculitis and auto-immune disease. Relative to the other subgroups, this subgroup presented a shorter median OS (8.6 months) and a shorter TTD. A possible explanation may be a greater toxicity of ICIs in this subgroup. Some authors have reported that patients with pre-existing granulomatosis with polyangiitis experience a flare-up of their disease under immunotherapy, which may compromise treatment persistence and survival.29,30 A retrospective study from the USA reported 56 patients with concurrent auto-immune disease and NSCLC of whom 18% had active underlying disease.31 Half of these patients experienced a flare-up of their pre-existing AID or an immune-related adverse event. However, our subgroup of patients with AID and granulomatosis consists of only 14 patients, so the findings should be interpreted with caution.
The second subgroup with intermediate OS (9.6 months) contains 59 patients with idiopathic ILD. To our knowledge, this is the first study to report on such a large number of these patients. In this group, IPF generally represents the main and most severe diagnosis with a prognosis of 3–5 years.32 Although, the pathogenesis of IPF is not fully understood, in vivo studies suggest a protective role of anti-programmed death1 / anti-programmed death ligand 1 (PD-1/PD-L1) activation in the fibrotic process.33 If this is the case, then administration of ICI could potentially worsen the underlying fibrosis and lead to more frequent exacerbations. In our study, the time to treatment discontinuation was not different from that of the general population, which could be interpreted as a sign of no excess exacerbation of the underlying disease. There are very few data on OS in this setting. A Japanese Phase II trial evaluating nivolumab in 18 patients with mild IPF reported a similar OS (15.6 [14.4–NR] months), without documenting significant excessive toxicities.16 On the other hand, the Japanese prospective trial with atezolizumab was stopped prematurely due to high pulmonary toxicity in the ILD group.17 The final analysis of clinical outcome in this study reported an OS of 15.3 months and a 1-year survival rate of 53.3%.34 In this ILD subgroup, prospective trials are mandatory in order to document the different risk factors with precision.
The third subgroup consists of all ILD cases with a known etiology, such as pneumoconiosis or hypersensitivity pneumonitis. This subgroup had almost the same outcome as the non-ILD cohort with a median OS of 10.7 months and a time to treatment discontinuation of 2.5 months. Our cohort is the first to focus on this category of patients as there are no data in the literature apart from one case report showing an exacerbation of bird fancier’s pneumonitis associated with hypersensitivity to pembrolizumab.35 Unfortunately, the study by Tasaka et al., which included a cohort of 49 patients of whom 65% did not have IPF, did not report survival or response rates by ILD aetiology. In our study, this subset of patients seemed to benefit from nivolumab to the same degree as patients without ILD.
The strengths of this study include the large number of patients with ILD evaluated, to our knowledge, the largest cohort of ILD patients treated with an ICI that has been reported to date. Secondly, the source UNIVOC cohort of >10,000 patients with aNSCLC can be considered well-validated, since it has been extensively characterized, followed for up to 5 years and demonstrates nivolumab treatment outcomes consistent with the pivotal clinical trials. The study also has certain limitations. For example, the comparison of outcomes between ILD and non-ILD patients should be interpreted with caution, as these two populations differed in several baseline characteristics that may influence outcome. A larger study adjusting for potential confounding factors would be necessary to demonstrate unequivocally that survival following nivolumab treatment is equivalent in the two groups of patients. As the data source is the PMSI, we have no access to patient medical records or information on the diagnostic criteria used or results of respiratory function tests or CT scan (such as honeycomb pattern), which are risk factors for acute exacerbations.36,37 For this reason, we are not able to confirm the severity of the ILD in the PMSI. Consequently, a possible selection bias is identified where only patients with mild ILD were offered treatment with nivolumab. These results are therefore helpful in terms of suggesting safety in patients with mild ILD in which therapy are already considered to be reasonably safety. Moreover, we have no data on the occurrence and the type of adverse events, which may be a critical determinant of outcome in patients with ILD. We can only postulate the occurrence of adverse event from early treatment discontinuation without knowing whether discontinuation is indeed due to an adverse event, and if so, which type of event. The data obtained do not any such suggest early discontinuation, except possibly for the AI/G subgroup. However, we suppose that the patients with ILD are selected ones, with potentially less severe or even favorable predictive biomarkers. Another limitation is that we do not have any data on concurrent medications and in particular on anti-fibrotic agents which are prescribed in IPF and other progressive fibrosing ILDs.38 In this context, some previous studies have highlighted a protective role for pirfenidone or nintedanib, in combination with chemotherapy or ICI, even when the patient had an history of immune-related ILD.39,40 Use of such medications may thus possibly modulate the risk of exacerbations under ICI therapy. Finally, it should be emphasized that the cohort only included patients treated with nivolumab as second or higher line for aNSCLC, and it may not be possible to generalize the findings to other ICIs or to the first-line setting.
Conclusions
Our cohort is the largest one published to date to report outcomes in patients with comorbid aNSCLC and ILD treated with nivolumab. We have shown nivolumab to offer a clinically meaningful benefit at least in certain patient subgroups. Our findings indicate that the aetiology of ILD may influence the treatment outcome, although the data should be interpreted with caution due to the small number of patients in certain ILD subgroups. Until a consensus on the use of immunotherapy in patients with ILD has been reached, the decision to prescribe ICIs to patients with ILD should be taken on a case-by-case basis from a multidisciplinary perspective. Dedicated investigational studies and prospective cohorts are critical in order to collect more data to characterize adequately the benefits and risks associated with ICI therapy in this patient population.
Supplemental Material
Supplemental material, sj-docx-1-tam-10.1177_17588359231152847 for Outcome following nivolumab treatment in patients with advanced non-small cell lung cancer and comorbid interstitial lung disease in a real-world setting by Jean-Baptiste Assié, Christos Chouaïd, Hilario Nunes, Dorothée Reynaud, Anne-Françoise Gaudin, Valentine Grumberg, Ronan Jolivel, Baptiste Jouaneton, François-Emery Cotté and Boris Duchemann in Therapeutic Advances in Medical Oncology
Acknowledgments
Writing and editorial assistance was provided by SARL Foxymed.
Footnotes
ORCID iD: Christos Chouaïd  https://orcid.org/0000-0002-4290-5524
https://orcid.org/0000-0002-4290-5524
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Jean-Baptiste Assié, Functional Genomics of Solid Tumors Laboratory, Centre de Recherche des Cordeliers, INSERM, Sorbonne Université, Université de Paris, Paris, France; Centre Hospitalier Intercommunal Créteil, Créteil, France.
Christos Chouaïd, Centre Hospitalier Intercommunal Créteil, Créteil, France.
Hilario Nunes, Department of Respiratory Medicine, Centre de Référence des Maladies Pulmonaires Rares, Avicenne Hospital, Université Sorbonne Paris Nord, Paris, France.
Dorothée Reynaud, Bristol Myers Squibb France, Rueil-Malmaison, France.
Anne-Françoise Gaudin, Bristol Myers Squibb France, Rueil-Malmaison, France.
Valentine Grumberg, Bristol Myers Squibb France, 3 rue Joseph Monier, Rueil-Malmaison 92500, France. Oncostat – U1018, INSERM, Paris-Saclay University, “Ligue Contre le Cancer” Labeled Team, Villejuif, France.
Ronan Jolivel, HEVA, Lyon, France.
Baptiste Jouaneton, HEVA, Lyon, France.
François-Emery Cotté, Bristol Myers Squibb France, Rueil-Malmaison, France.
Boris Duchemann, Department of Thoracic and Medical Oncology, Avicenne Hospital, Université Sorbonne Paris Nord, Paris, France; Laboratoire d’Immunomonitoring en Oncologie, INSERM US23, CNRS UMS 3655, Institut Gustave Roussy, Villejuif, France.
Declarations
Ethical approval and consent to participate: The study was performed according to Good Pharmacoepidemiology Practice (GPP) and relevant local and international regulatory requirements. Since this was a retrospective study of an anonymized database and had no influence on patient care, ethics committee approval was not required, according to French law. The study was performed according to the MR006 guideline of the French data protection agency.
Consent for publication: Not applicable.
Author contribution(s): Jean-Baptiste Assié: Conceptualization; Formal analysis; Investigation; Methodology; Writing – original draft; Writing – review & editing.
Christos Chouaïd: Conceptualization; Formal analysis; Investigation; Methodology; Writing-review & editing.
Hilario Nunes: Conceptualization; Formal analysis; Investigation; Methodology; Writing-review & editing.
Dorothée Reynaud: Data curation; Formal analysis; Investigation; Methodology; Writing-review & editing.
Anne-Françoise Gaudin: Conceptualization; Writing-review & editing.
Valentine Grumberg: Conceptualization; Data curation; Formal analysis; Investigation; Project administration; Writing – original draft; Writing – review & editing.
Ronan Jolivel: Data curation; Formal analysis.
Baptiste Jouaneton: Data curation; Formal analysis.
François-Emery Cotté: Conceptualization; Writing – original draft; Writing – review & editing.
Boris Duchemann: Conceptualization; Writing – original draft; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by Bristol Myers Squibb (Princeton, NJ) and ONO Pharmaceutical Company Ltd. (Osaka, Japan).
VG, CC, FEC, AFG, and DR are employees of BMS. BJ and RJ are employees of HEVA. JBA, HN, and CC report no conflict of interest. BD reports personal fees for medical training for BMS, Roche, Pfizer, Astra Zeneca, Chiesi, Amgen, Lilly and congress fees from Astra Zeneca, Pfizer and Oxyvie.
Availability of data and materials: The source database (PMSI) contains personal health data with potentially identifying and sensitive patient information. According to French law (Decree N° 2016-1871 dated 28th December 2016, concerning the processing of personal data in the SNDS), PMSI data are available exclusively from the database holder, the CNAMTS (Caisse nationale de l’assurance maladie des travailleurs salariés), to institutions who meet the criteria for access to confidential data, following procurement of consent from the National Health Data Institute (INDS) and the French data protection authority (CNIL). Publication of individual patient data is not permitted. The INDS, which is responsible for access to health data in France, is a one-stop-shop window for access to the SNDS database (including the PMSI database). The contact address for the INDS is Institut National des Données de Santé (INDS), 19 rue Arthur Croquette, 94220 Charenton-le-Pont, Telephone: +33 1 45 18 43 90; Email: contact@indsante.fr; Website: https://www.indsante.fr/fr.
References
- 1. Defossez G, Le Guyader-Peyrou S, Uhry Z, et al. Estimations nationales de l’incidence et de la mortalité par cancer en France métropolitaine entre 1990 et 2018. Étude à partir des registres des cancers du réseau Francim. Volume 1 - Tumeurs solides. Compléments par localisation tumorale et sous-sites ou sous-types histologiques. Saint-Maurice: Santé Publique France; 2019. [Google Scholar]
- 2. Defossez G, Uhry Z, Delafosse P, et al. Cancer incidence and mortality trends in France over 1990–2018 for solid tumors: the sex gap is narrowing. BMC Cancer 2021; 21: 726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015; 373: 123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015; 373: 1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Borghaei H, Gettinger S, Vokes EE, et al. Five-year outcomes from the randomized, phase III trials checkmate 017 and 057: nivolumab versus docetaxel in previously treated non-small-cell lung cancer. J Clin Oncol 2021; 39: 723–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barlesi F, Dixmier A, Debieuvre D, et al. Effectiveness and safety of nivolumab in the treatment of lung cancer patients in France: preliminary results from the real-world EVIDENS study. Oncoimmunology 2020; 9: 1744898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Giaj Levra M, Cotte FE, Corre R, et al. Immunotherapy rechallenge after nivolumab treatment in advanced non-small cell lung cancer in the real-world setting: a national data base analysis. Lung Cancer 2020; 140: 99–106. [DOI] [PubMed] [Google Scholar]
- 8. Assie JB, Corre R, Levra MG, et al. Nivolumab treatment in advanced non-small cell lung cancer: real-world long-term outcomes within overall and special populations (the UNIVOC study). Ther Adv Med Oncol 2020; 12: 1758835920967237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Debieuvre D, Juergens RA, Asselain B, et al. Two-year survival with nivolumab in previously treated advanced non-small-cell lung cancer: a real-world pooled analysis of patients from France, Germany, and Canada. Lung Cancer 2021; 157: 40–47. [DOI] [PubMed] [Google Scholar]
- 10. Duchemann B, Annesi-Maesano I, Jacobe de, Naurois C, et al. Prevalence and incidence of interstitial lung diseases in a multi-ethnic county of Greater Paris. Eur Respir J 2017; 50: 1602419. [DOI] [PubMed] [Google Scholar]
- 11. Whittaker Brown SA, Padilla M, Mhango G, et al. Interstitial lung abnormalities and lung cancer risk in the National Lung Screening Trial. Chest 2019; 156: 1195–1203. [DOI] [PubMed] [Google Scholar]
- 12. Naccache JM, Gibiot Q, Monnet I, et al. Lung cancer and interstitial lung disease: a literature review. J Thorac Dis 2018; 10: 3829–3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tzouvelekis A, Antoniou K, Kreuter M, et al. The DIAMORFOSIS (diagnosis and management of lung cancer and fibrosis) survey: international survey and call for consensus. ERJ Open Res 2021; 7: 00529–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yamaguchi T, Shimizu J, Hasegawa T, et al. Pre-existing pulmonary fibrosis is a risk factor for anti-PD-1-related pneumonitis in patients with non-small cell lung cancer: a retrospective analysis. Lung Cancer 2018; 125: 212–217. [DOI] [PubMed] [Google Scholar]
- 15. Fujimoto D, Morimoto T, Ito J, et al. A pilot trial of nivolumab treatment for advanced non-small cell lung cancer patients with mild idiopathic interstitial pneumonia. Lung Cancer 2017; 111: 1–5. [DOI] [PubMed] [Google Scholar]
- 16. Fujimoto D, Yomota M, Sekine A, et al. Nivolumab for advanced non-small cell lung cancer patients with mild idiopathic interstitial pneumonia: a multicenter, open-label single-arm phase II trial. Lung Cancer 2019; 134: 274–278. [DOI] [PubMed] [Google Scholar]
- 17. Ikeda S, Kato T, Kenmotsu H, et al. A phase 2 study of atezolizumab for pretreated NSCLC with idiopathic interstitial pneumonitis. J Thorac Oncol 2020; 15: 1935–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Saito S, Lasky JA, Hagiwara K, et al. Ethnic differences in idiopathic pulmonary fibrosis: the Japanese perspective. Respir Investig 2018; 56: 375–383. [DOI] [PubMed] [Google Scholar]
- 19. Duchemann B, Pluvy J, Crestani B, et al. Immune checkpoint blockade for patients with lung cancer and idiopathic pulmonary fibrosis. Eur J Cancer 2021; 145: 179–182. [DOI] [PubMed] [Google Scholar]
- 20. Cortellini A, Mallardo D, Cleary S, et al. Diabetes therapy burden as proxy of impairment of immune checkpoint inhibitors efficacy. Ann Oncol 2021; 32: S834. [Google Scholar]
- 21. Tasaka Y, Honda T, Nishiyama N, et al. Non-inferior clinical outcomes of immune checkpoint inhibitors in non-small cell lung cancer patients with interstitial lung disease. Lung Cancer 2021; 155: 120–126. [DOI] [PubMed] [Google Scholar]
- 22. Honda T, Hiroyuki S, Masai K, et al. Deleterious pulmonary surfactant system gene mutations in lung adenocarcinomas associated with usual interstitial pneumonia. JCO Precis Oncol 2018; 2: 1–24. [DOI] [PubMed] [Google Scholar]
- 23. Shibaki R, Murakami S, Matsumoto Y, et al. Tumor expression and usefulness as a biomarker of programmed death ligand 1 in advanced non-small cell lung cancer patients with preexisting interstitial lung disease. Med Oncol 2019; 36: 49. [DOI] [PubMed] [Google Scholar]
- 24. Fujimoto D, Sato Y, Morimoto T, et al. Programmed cell death ligand 1 expression in non-small-cell lung cancer patients with interstitial lung disease: a matched case-control study. Clin Lung Cancer 2018; 19: e667–e673. [DOI] [PubMed] [Google Scholar]
- 25. Duchemann B, Remon J, Naigeon M, et al. Current and future biomarkers for outcomes with immunotherapy in non-small cell lung cancer. Transl Lung Cancer Res 2021; 10: 2937–2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Haratani K, Hayashi H, Chiba Y, et al. Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol 2018; 4: 374–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kudoh S, Kato H, Nishiwaki Y, et al. Interstitial lung disease in Japanese patients with lung cancer: a cohort and nested case-control study. Am J Respir Crit Care Med 2008; 177: 1348–1357. [DOI] [PubMed] [Google Scholar]
- 28. INSEE. 2022. https://www.insee.fr/fr/statistiques/2381750#graphique-figure1_radio1.
- 29. Sibille A, Alfieri R, Malaise O, et al. Granulomatosis with polyangiitis in a patient on programmed death-1 inhibitor for advanced non-small-cell lung cancer. Front Oncol 2019; 9: 478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nabel CS, Severgnini M, Hung YP, et al. Anti-PD-1 immunotherapy-induced flare of a known underlying relapsing vasculitis mimicking recurrent cancer. Oncologist 2019; 24: 1013–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Leonardi GC, Gainor JF, Altan M, et al. Safety of programmed death-1 pathway inhibitors among patients with non-small-cell lung cancer and preexisting autoimmune disorders. J Clin Oncol 2018; 36: 1905–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Raghu G, Remy-Jardin M, Myers JL, et al. Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med 2018; 198: e44–e68. [DOI] [PubMed] [Google Scholar]
- 33. Ni K, Liu M, Zheng J, et al. PD-1/PD-L1 pathway mediates the alleviation of pulmonary fibrosis by human mesenchymal stem cells in humanized mice. Am J Respir Cell Mol Biol 2018; 58: 684–695. [DOI] [PubMed] [Google Scholar]
- 34. Sato Y, Ikeda S, Kato T, et al. Final analysis of TORG1936/AMBITIOUS: phase II study of atezolizumab for pretreated non-small cell lung cancer with idiopathic interstitial pneumonia. Ann Oncol 2021; 32: S998–S999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vu A, Vassallo R, Ryu JH. Exacerbation of previously undiagnosed bird fancier’s lung by pembrolizumab therapy. Chest 2019; 155: e79–e82. [DOI] [PubMed] [Google Scholar]
- 36. Kenmotsu H, Naito T, Kimura M, et al. The risk of cytotoxic chemotherapy-related exacerbation of interstitial lung disease with lung cancer. J Thorac Oncol 2011; 6: 1242–1246. [DOI] [PubMed] [Google Scholar]
- 37. Enomoto Y, Inui N, Kato T, et al. Low forced vital capacity predicts cytotoxic chemotherapy-associated acute exacerbation of interstitial lung disease in patients with lung cancer. Lung Cancer 2016; 96: 63–67. [DOI] [PubMed] [Google Scholar]
- 38. Raghu G, Rochwerg B, Zhang Y, et al. An official ATS/ERS/JRS/ALAT clinical practice guideline: treatment of idiopathic pulmonary fibrosis. An update of the 2011 clinical practice guideline. Am J Respir Crit Care Med 2015; 192: e3–e19. [DOI] [PubMed] [Google Scholar]
- 39. Yamakawa H, Oba T, Ohta H, et al. Nintedanib allows retreatment with atezolizumab of combined non-small cell lung cancer/idiopathic pulmonary fibrosis after atezolizumab-induced pneumonitis: a case report. BMC Pulm Med 2019; 19: 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yamamoto Y, Yano Y, Kuge T, et al. Safety and effectiveness of pirfenidone combined with carboplatin-based chemotherapy in patients with idiopathic pulmonary fibrosis and non-small cell lung cancer: a retrospective cohort study. Thorac Cancer 2020; 11: 3317–3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tam-10.1177_17588359231152847 for Outcome following nivolumab treatment in patients with advanced non-small cell lung cancer and comorbid interstitial lung disease in a real-world setting by Jean-Baptiste Assié, Christos Chouaïd, Hilario Nunes, Dorothée Reynaud, Anne-Françoise Gaudin, Valentine Grumberg, Ronan Jolivel, Baptiste Jouaneton, François-Emery Cotté and Boris Duchemann in Therapeutic Advances in Medical Oncology