Abstract
Mechanical and metabolic signals associated with skeletal muscle contraction stimulate the sensory endings of thin fibre muscle afferents, which, in turn, generates reflex increases in sympathetic nerve activity (SNA) and blood pressure (the exercise pressor reflex; EPR). EPR activation in patients and animals with heart failure with reduced ejection fraction (HF-rEF) results in exaggerated increases in SNA and promotes exercise intolerance. In the healthy decerebrate rat, a subtype of acid sensing ion channel (ASIC) on the sensory endings of thin fibre muscle afferents, namely ASIC1a, has been shown to contribute to the metabolically sensitive portion of the EPR (i.e. metaboreflex), but not the mechanically sensitive portion of the EPR (i.e. the mechanoreflex). However, the role played by ASIC1a in evoking the EPR in HF-rEF is unknown. We hypothesized that, in decerebrate, unanaesthetized HF-rEF rats, injection of the ASIC1a antagonist psalmotoxin-1 (PcTx-1; 100 ng) into the hindlimb arterial supply would reduce the reflex increase in renal SNA (RSNA) evoked via 30 s of electrically induced static hindlimb muscle contraction, but not static hindlimb muscle stretch (model of mechanoreflex activation isolated from contraction-induced metabolite-production). We found that PcTx-1 reduced the reflex increase in RSNA evoked in response to muscle contraction (n = 8; mean (SD) ∫ΔRSNA pre: 1343 (588) a.u.; post: 816 (573) a.u.; P = 0.026) and muscle stretch (n = 6; ∫ΔRSNA pre: 688 (583) a.u.; suggest post: 304 (370) a.u.; P = 0.025). Our data that, in HF-rEF rats, ASIC1a contributes to activation of the exercise pressor reflex and that contribution includes a novel role for ASIC1a in mechanosensation that is not present in healthy rats.
Keywords: ASICs, exercise tolerance, heart failure reduced ejection fraction, mechanoreflex, metaboreflex, muscle afferents, sympatho-excitation
Graphical Abstract
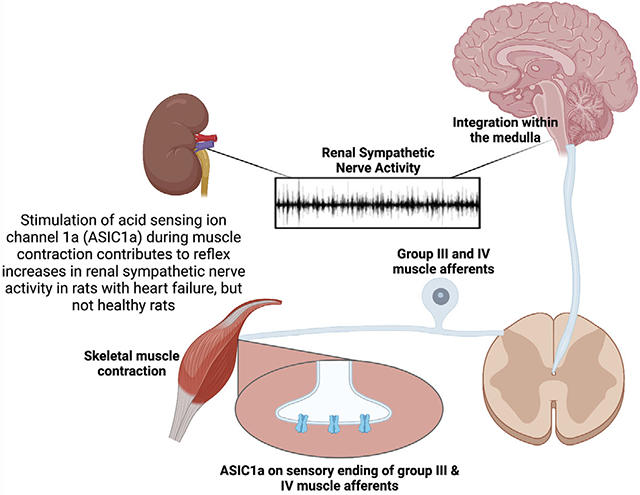
Acid-sensing ion channel 1a (ASIC1a) on the sensory endings of thin fibre skeletal muscle afferents contributes to the reflex increase in renal sympathetic nerve activity (RSNA) during skeletal muscle contractions in rats with heart failure, but not healthy rats.
Introduction
During exercise, a withdrawal of parasympathetic nervous system activity and an increase in sympathetic nervous system activity (SNA) facilitates numerous cardiovascular adjustments, which include increases in heart rate (HR), cardiac contractility and blood pressure (BP; Kaufman & Forster, 1996). The exercise pressor reflex is a feedback autonomic control mechanism that contributes importantly to those exercise-induced changes in autonomic activity (Amann, Runnels et al., 2011; Kaufman & Forster, 1996; Kaufman et al., 1983; McCloskey & Mitchell, 1972; Mitchell et al., 1983; Strange et al., 1993). The reflex is activated when the sensory endings of thinly myelinated group III and unmyelinated group IV skeletal muscle afferents (collectively termed thin fibre muscle afferents) are stimulated by mechanical and/or metabolic signals associated with muscle contraction (Kaufman et al., 1982, 1983, 1984; McCloskey & Mitchell, 1972; Mense & Stahnke, 1983; Mense & Meyer, 1988; Rotto & Kaufman, 1988).
A family of acid sensing ion channels (ASICs) distributed throughout the central and peripheral nervous systems plays an important role in detecting acidic stimuli and regulating the cardiovascular system (Abboud & Benson, 2015). On the sensory endings of thin fibre muscle afferents, ASICs principally form heterotrimeric channels composed of ASIC1a, ASIC2 and ASIC3 subunits (Gautam & Benson, 2013). During exercise, ASICs are stimulated by acidic products of skeletal muscle contraction, which contributes importantly to the activation of the metabolic portion of the exercise pressor reflex (McCord et al., 2009; Victor et al., 1988) (i.e. the metaboreflex). In support of this notion, local infusion/injection of the non-selective ASIC inhibitor amiloride attenuated the pressor and MSNA response to static handgrip exercise in healthy humans (Campos et al., 2019) as well as the pressor and thin fibre muscle afferent response to static hindlimb muscle contraction in healthy cats (Hayes et al., 2007; McCord et al., 2008, 2009). Ducrocq et al. (2020a, 2020b) recently extended those findings by demonstrating that local selective ASIC1a blockade attenuated the pressor response to electrically induced static hindlimb muscle contraction in healthy rats. The remaining two subclasses of ASICs present on the sensory endings of thin fibre muscle afferents are either activated via noxious pH levels not likely present during skeletal muscle contraction (i.e. ASIC2 with a pH50 of 4.5; Hesselager et al., 2004) or, in the case of ASIC3, have been shown to play little (Tsuchimochi et al., 2011) or no (Kim et al., 2019, 2020; Stone et al., 2015) independent role in evoking the exercise pressor reflex in healthy rats. Thus, it appears that ASIC1a, but not ASIC2 or ASIC3, plays a key role in evoking the exercise pressor reflex, at least in the healthy rat.
In comparison to healthy subjects, activation of the exercise pressor reflex in patients/animals with heart failure with reduced ejection fraction (HF-rEF) results in an exaggerated increase in SNA (Butenas et al., 2021c; Koba et al., 2008; Middlekauff et al., 2004; Wang, Pan et al., 2010) and altered cardiovascular responses (Amann et al., 2014; Ansorge et al., 2004; Hammond et al., 2000; Piepoli et al., 1996; Sterns et al., 1991), which include augmented peripheral (Amann et al., 2014; Smith et al., 2020) and coronary (Ansorge et al., 2004) vasoconstriction. Thus, whereas activation of the exercise pressor reflex in healthy subjects supports exercise performance (Amann, Blain et al., 2011; O’Leary et al., 1999), activation of the reflex in HF-rEF patients likely contributes to exacerbated fatigue, exercise intolerance and elevated cardiovascular risk.
In HF-rEF, the accumulation of acidic products of skeletal muscle contraction is greater than that found during skeletal muscle contraction in healthy subjects (Arnolda et al., 1991). For example, in HF-rEF patients muscle pH decreases and lactate ions increase to a greater extent during exercise compared to the changes found in healthy control subjects (Massie et al., 1988), an effect which may produce greater ASIC stimulation during exercise in HF-rEF patients. A role for ASIC3 in the exaggerated exercise pressor reflex in HF-rEF seems unlikely given the findings of Xing et al. (2015) that, compared to healthy control rats, HF-rEF rats had lower ASIC3 protein expression in L4/L5 dorsal root ganglia (DRG) tissue and a lower percentage of DRG neurons expressing ASIC3-like currents. ASIC1a, however, is an appealing candidate as a possible contributor to the exaggerated exercise pressor reflex in HF-rEF. For example, HF-rEF is a proinflammatory condition (Murphy et al., 2020) and experimentally induced inflammation has been shown to increase ASIC1a expression (Voilley et al., 2001) and potentiate ASIC1a responsiveness (Smith et al., 2007) in rat sensory neurons. Unfortunately, however, the role played by ASIC1a in evoking the exercise pressor reflex in HF-rEF has not been investigated.
Based on the information above, the purpose of this investigation was to determine the role played by ASIC1a on the sensory endings of group III/IV muscle afferents in evoking the exercise pressor reflex in a myocardial infarction (MI)-induced rat model of HF-rEF. Specifically, we tested the hypothesis that, in decerebrate, unanaesthetized rats, hindlimb arterial injection of the ASIC1a antagonist psalmotoxin-1 (PcTx-1) would reduce the reflex increase in renal SNA (RSNA), BP and HR evoked in response to 30 s of electrically induced static hindlimb skeletal muscle contraction to a greater extent in HF-rEF rats when compared to sham-operated healthy control rats (SHAM rats). We also tested the hypothesis that ASIC1 mRNA and ASIC1a protein expression in L4/L5 DRG tissue would be greater in HF-rEF rats compared to SHAM rats.
Methods
Ethical approval
All experimental procedures were approved by the Institutional Animal Care and Use Committee of Kansas State University (protocol no. 4552) and conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (2011). Experiments were performed on ~14- to 19-week-old male Sprague–Dawley rats (n = 102; Charles River Laboratories). Rats were housed two per cage in temperature (maintained at ~22°C) and light (12 h–12 h light–dark cycle running from 07.00 to 19.00 h)-controlled accredited facilities with standard rat chow and water provided ad libitum.
Surgical procedure
Myocardial infarction (MI) was induced in 59 of the 102 rats by surgically ligating the left main coronary artery (Musch & Terrell, 1992). Briefly, rats were anaesthetized initially with a 5% isoflurane–O2 mixture (Butler Animal Health Supply, Elk Grove Village, IL, USA, and Linweld, Dallas, TX, USA) and maintained subsequently on 2.5% isoflurane–O2 and then intubated and mechanically ventilated with a rodent respirator (Harvard model 680, Harvard Instruments, Holliston, MA, USA) for the duration of the surgical procedure. After a single injection of the antiarrhythmic drug amiodarone (100 mg/kg i.p.), which was administered to improve survival rate following the ensuing coronary artery ligation surgery (Kolettis et al., 2007), a left thoracotomy was performed to expose the heart through the fifth intercostal space, and the left main coronary artery was ligated 1–2 mm distal to the edge of the left atrium with a 6–0 braided polyester suture. The thorax was then closed with 2–0 gut, and the skin was closed with 2–0 silk. Prior to termination of anaesthesia, bupivacaine (1.5 mg/kg s.c.) and buprenorphine (~0.03 mg/kg i.m.) were administered to reduce pain associated with the surgery, along with ampicillin (50 mg/kg i.m.) to reduce the risk of infection. After rats were removed from mechanical ventilation and anaesthesia, they were monitored closely for ~6 h post-surgery. In the remaining 43 of 102 rats, a sham ligation of the coronary artery was performed in which 6–0 braided polyester suture was passed under the left main coronary artery, but not tied. These rats are referred to as ‘SHAM’ rats from this point forward. Following completion of either MI or SHAM procedures, rats were housed one per cage for 10 days to minimize risk of infection of the surgical site. During these 10 days, the antibiotic Baytril (100 mg/ml) was administered in the drinking water. Following completion of the Baytril treatment, rats were housed two per cage as described above. All animals were monitored daily for 14 days following the MI or SHAM procedure for changes in behaviour, gait/posture, breathing, appetite and body weight.
Echocardiograph measurements
Transthoracic echocardiograph measurements were performed with a commercially available system (LOGIQ e; GE Health Care, Milwaukee, WI, USA) no more than 1 week before the final experimental protocol. Briefly, the rats were anaesthetized as described above. Once the rat was fully anesthetized, the isoflurane mixture was reduced to 2.5% isoflurane–O2. Following 5 min at 2.5% isoflurane, echocardiograph measurements began. The transducer was positioned on the left anterior chest, and left ventricular dimensions were measured. The left ventricular fractional shortening (FS), ejection fraction (EF), end diastolic (LVEDV), end systolic volume (LVESV), and stroke volume (SV) were determined by echocardiographic measurements as previously described (Baumfalk et al., 2021). Rats with HF-rEF were required to meet an inclusion criterion of an FS ≤ 30% and/or a left ventricular infarct size of ≥15% (see below for method of infarct size determination), which is consistent with the criterion previously established by our laboratory (Butenas et al., 2020, 2021a, 2021b). We have previously shown that, compared with SHAM operated healthy control rats, HF-rEF rats that meet similar criterion have a reduced maximal oxygen uptake and time to exhaustion during treadmill running (Butenas, et al., 2021a).
Surgical procedures for experimental protocols
In vivo experiments were performed on 69 rats (30 SHAM, 39 HF-rEF) between 6 and 8 weeks following the MI or SHAM procedure. Importantly, this time frame post-MI procedure ensures the development of HF-rEF as well as reduced maximal oxygen uptake and time to exhaustion during treadmill running (Butenas, et al., 2021a). On the day of the experiment, rats were anesthetized as described above. Adequate depth of anaesthesia was confirmed by the absence of toe-pinch and blink reflexes. The trachea was cannulated, and the lungs were mechanically ventilated (Harvard Apparatus) with a 2% isoflurane–balance O2 gaseous mixture until the decerebration was completed (see below). The right jugular vein and both carotid arteries were cannulated with PE-50 catheters, which were used for the injection of fluids, measurement of arterial blood pressure (physiological pressure transducer, ADInstruments, Colorado Springs, CO, USA), and sampling of arterial blood gasses (Radiometer, Brea, CA, USA). HR was calculated from the R–R interval measured by electrocardiogram (ADInstruments). The left superficial epigastric artery was cannulated with a PE-8 catheter whose tip was placed near the junction of the superficial epigastric and femoral arteries. A reversible snare was placed around the left iliac artery and vein (i.e. proximal to the location of the catheter placed in the superficial epigastric artery). The left calcaneal bone was severed and linked by string to a force transducer (Grass FT03; Grass Instrument Co, West Warwick, RI, USA), which, in turn, was attached to a rack and pinion. A ~1–2 cm section of the left sciatic nerve was exposed by reflecting the overlaying skeletal muscles.
Upon completion of the initial surgical procedures, rats were placed in a Kopf stereotaxic frame. After administering dexamethasone (0.2 mg i.v.) to minimize swelling of the brainstem, a pre-collicular decerebration was performed in which all brain tissue rostral to the superior colliculi was removed (Smith et al., 2001). The cranial cavity was filled with cotton balls and covered with glue (Kwik-Sil, World Precision Instruments, Sarasota, FL, USA). Following decerebration, anaesthesia was reduced to 0.5%. A retroperitoneal approach was used to expose bundles of the left renal sympathetic nerve, which were then glued with Kwik-Sil onto a pair of thin stainless-steel recording electrodes connected to a high impedance probe (Grass Model HZP) and amplifier (Grass P511). Multiunit signals from the renal sympathetic nerve fibres were filtered at high and low frequencies (1 kHz and 100 Hz, respectively) for the measurement of RSNA.
Upon completion of all surgical procedures, anaesthesia was terminated, and the rats’ lungs were ventilated with room air. Experimental protocols commenced at least 1 h after termination of isoflurane. Experiments were performed on decerebrate, unanaesthetized rats because anaesthesia has been shown to markedly blunt the exercise pressor reflex in the rat (Smith et al., 2001). Body core temperature was measured via a rectal probe and maintained at ~37–38°C by an automated heating system (Harvard Apparatus) and heat lamp. Arterial pH and blood gases were analysed periodically throughout the experiment from arterial blood samples (~75 μl) and maintained within physiological ranges (pH: 7.35–7.45, PCO2: ~38–40 mmHg, PO2: ~100 mmHg) by administration of sodium bicarbonate and/or adjusting ventilation as necessary. At the end of all experiments in which an experimental solution was injected into the arterial supply of the left hindlimb through the superficial epigastric artery catheter, Evans blue dye was injected in the same manner as the experimental solution to confirm that the injectate had access to the triceps surae muscle circulation. The triceps surae muscles were observed to stain blue in all experiments. Then, postganglionic sympathetic nerve activity was abolished with administration of hexamethonium bromide (10 mg; 0.5 ml saline i.v.) to allow for the quantification of background noise as described previously (Kempf et al., 2018). Rats were then anaesthetized with 5% isoflurane and humanely euthanized with an injection of potassium chloride (>3 mg/kg i.a.). A pneumothorax was then performed, and the heart was excised. The atria and right ventricle (RV) were separated from the left ventricle (LV) and septum, and the RV, LV and atria were weighed. In rats with HF-rEF, the LV infarction surface area was measured using planimetry and expressed as percentage of LV end-ocardial surface area as described previously (Craig et al., 2019).
Lactic acid injection protocol
In 23 rats (12 SHAM, 11 HF-rEF), we attempted to confirm the efficacy of PcTx-1 in blocking ASIC1a on the sensory endings of thin fibre muscle afferents by reproducing the previous finding from Ducrocq et al. (2020b) that hindlimb arterial injection of PcTx-1 (100 ng; 0.1 ml saline) reduced the pressor and cardioaccelerator response to hindlimb arterial injection of lactic acid (24 mmol; 0.2 ml saline). Briefly, the lactic acid solution was injected as a bolus into the arterial supply of the left hindlimb through the superficial epigastric artery catheter. Following a ~5 min recovery, the snare around the left iliac artery and vein was tightened and PcTx-1 was injected into the arterial supply of the hindlimb through the superficial epigastric artery catheter. The snare was released 5 min after injecting PcTx-1 and, following a 5 min recovery period, a second lactic acid injection was performed. Evans blue dye was injected in the same manner as the lactic acid and PcTx-1 and the triceps surae muscles were observed to stain blue in all experiments.
Effect of ASIC1a blockade on the exercise pressor reflex
In 15 rats (7 SHAM, 8 HF-rEF), we compared the renal sympathetic, pressor, and cardioaccelerator responses evoked in response to 30 s of static hindlimb muscle contraction before and after the injection of PcTx-1 into the arterial supply of the left hindlimb. Following recovery from isoflurane anaesthesia, baseline muscle tension was set to ~100 g and baseline RSNA, blood pressure and HR were measured for ~30 s. The sciatic nerve was then electrically stimulated using stainless steel electrodes for 30 s at a voltage of ~1.5 × motor threshold (0.01 ms pulse duration, 40 Hz frequency), which produced static contractions of the triceps surae muscles. Approximately 10 min following the control contraction manoeuvre, the snare on the left iliac artery and vein was tightened and PcTx-1 was injected into the femoral artery using the superficial epigastric artery catheter as described above. PcTx-1 remained snared in the hindlimb circulation for 5 min, at which time the iliac snare was released. The hindlimb was reperfused for 5 min and the contraction manoeuvre was then repeated exactly as described above. At the end of all experiments, we injected the paralytic pancuronium bromide (0.5 mg; 0.5 ml saline i.v.) and the sciatic nerve was stimulated for 30 s with the same parameters as those used to elicit contraction to ensure that the increase in RSNA, blood pressure and HR during contraction was not due to the electrical activation of the axons of the thin fibre muscle afferents in the sciatic nerve. No increase in RSNA, blood pressure or HR was observed during the stimulation period following the administration of pancuronium bromide.
Control for possible effect of vehicle for PcTx-1 on the exercise pressor reflex
In seven rats (3 SHAM, 4 HF-rEF), we compared the renal sympathetic, pressor and cardioaccelerator responses evoked in response to 30 s of contraction before and after the injection of the vehicle for PcTx-1 (i.e. 0.1 ml saline) into the catheter placed in the superficial epigastric artery. Successful RSNA recordings were accomplished in all but three of these rats (two SHAM, one HF-rEF). These three rats were used in the vehicle control protocols to maximize the statistical power of the RSNA comparisons in the main experimental protocols.
Control for possible blockade of ASIC1a within the central nervous system
PcTx-1 is a large molecule (4689 Da) and therefore unlikely able to cross the blood–brain barrier (pore size of ~600 Da) and exert an effect in the central nervous system (Dibas et al., 2019). Nevertheless, we investigated the possibility that systemic circulation of PcTx-1 and consequent inhibition of ASIC1a within the central nervous system (e.g. spinal cord, brain stem) may have accounted for the attenuating effects in the main experimental group in which PcTx-1 was injected into the hindlimb arterial circulation rather than a local effect within the hindlimb. In nine rats (5 SHAM, 4 HF-rEF), we compared the renal sympathetic, pressor and cardioaccelerator responses evoked in response to 30 s of contraction before and after the injection of PcTx-1 into the catheter placed in the jugular vein. Successful RSNA recordings were accomplished in all but three of these rats (2 SHAM, 1 HF-rEF). These three rats were used in the systemic control protocols to maximize the statistical power of the RSNA comparisons in the main experimental protocols.
Effect of ASIC1a blockade on isolated mechanoreflex activation
In separate groups of SHAM (n = 6) and HF-rEF rats (n = 7), we investigated the possibility that the effect of ASIC1a blockade with PcTx-1 on the RSNA, pressor, and cardioaccelerator response was attributed to an effect on the mechanoreflex. Specifically, we compared the RSNA, pressor, and cardioaccelerator response to static hindlimb muscle stretch, a model of static mechanoreflex activation isolated from contraction-induced metabolite production (Stebbins et al., 1988) before and after the injection of PcTx-1 into the arterial supply of the left hindlimb. Following recovery from isoflurane anaesthesia, baseline muscle tension was set to ~100 g and baseline RSNA, blood pressure and HR were measured for ~30 s. An experienced investigator then initiated the stretch protocol by rapidly turning the rack and pinion to a position which was held constant for 30 s. The investigator aimed to produce a peak tension generation of 1.0 kg during each static stretch to match the average tension developed in the contraction experiments in the present investigation. Approximately 5 min following the control stretch manoeuvre, the snare on the left iliac artery and vein was tightened and PcTx-1 was injected into the femoral artery using the superficial epigastric artery catheter as described above. PcTx-1 remained snared in the hindlimb circulation for 5 min, at which time the iliac snare was released. The hindlimb was reperfused for 5 min and the static stretch manoeuvre was then repeated exactly as described above. The tension generated during the post-drug manoeuvres was matched as closely as possible to that produced during the control stretch.
Control for the possible effect of PcTx-1 activating an endogenous enkephalin pathway
In five HF-rEF rats we compared the renal sympathetic, pressor, cardioaccelerator responses to 30 s of static hindlimb muscle stretch before and after μ- and δ-opioid receptor and then after ASIC1a blockade. These experiments served as a control for the possibility that the effect of ASIC1a blockade with PcTx-1 on the integrated renal sympathetic response to static hindlimb muscle stretch (Figs 5B and 6B) was attributed to a release of Met-enkephalin, a μ- and δ-opioid receptor agonist (Mazzuca et al., 2007). During these experiments, a control stretch manoeuvre was performed exactly as described above. Next, following a ~5 min recovery period, naloxone (100 μg; 0.1 ml saline), a μ- and δ-opioid receptor antagonist, was infused at a constant rate of 10 μl/min for 10 min into the hindlimb circulation via the catheter placed in the superficial epigastric artery as described previously (Tsuchimochi et al., 2010). Approximately 5 min following injection of naloxone, a second stretch manoeuvre was performed. Next, after a second ~5 min recovery period, the snare on the left iliac artery and vein was tightened and PcTx-1 was injected into the femoral artery using the superficial epigastric artery catheter as described above. PcTx-1 remained snared in the hindlimb circulation for 5 min, at which time the iliac snare was released. The hindlimb was reperfused for 5 min and the static stretch manoeuvre was then repeated exactly as described above.
Figure 5. Effect of ASIC1a blockade on the time course of mechanoreflex activation.
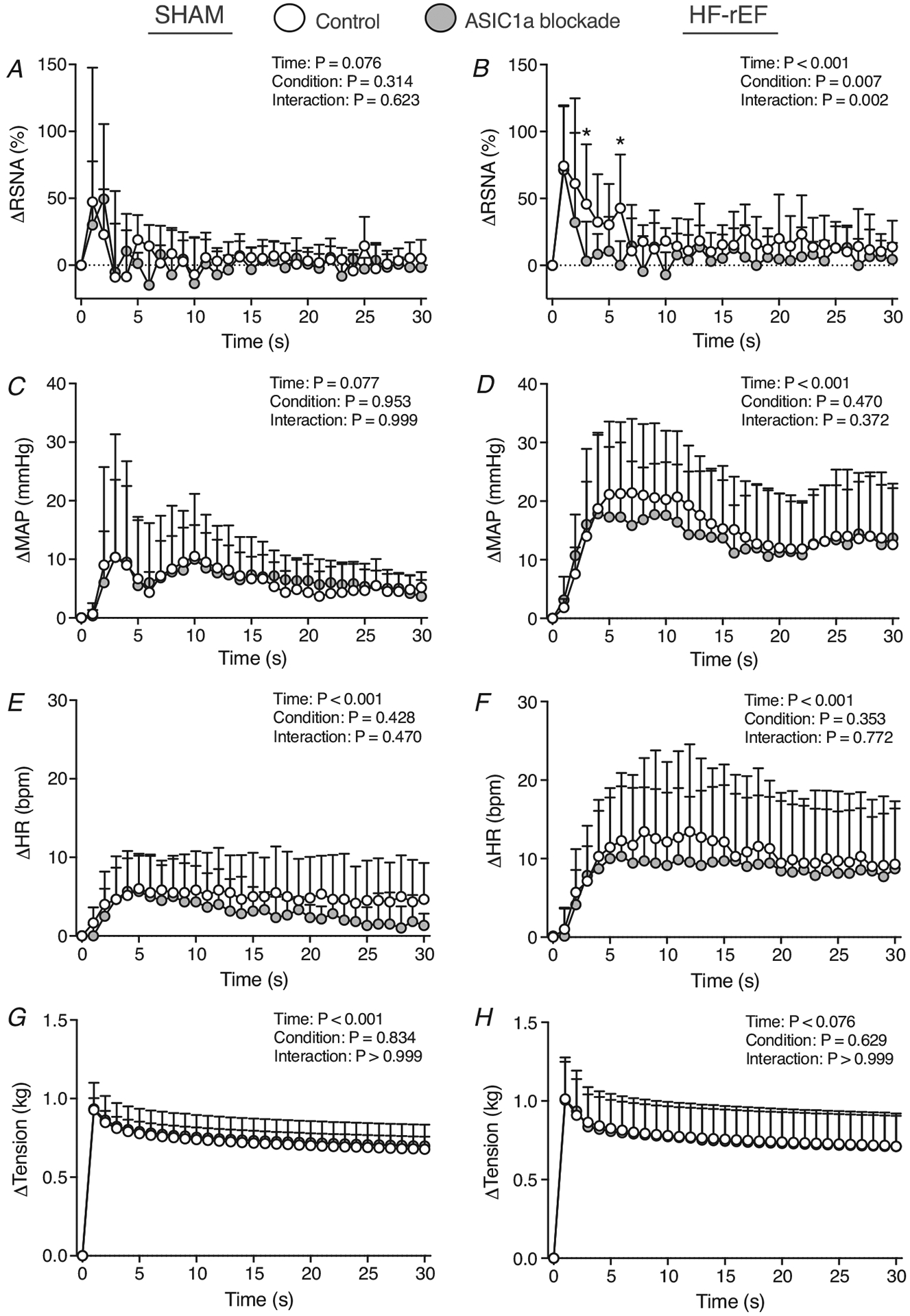
Δ renal sympathetic nerve activity (RSNA; A and B), Δ mean arterial pressure (MAP; C and D), Δ heart rate (HR; E and F), and tension (G and H) in response to 30 s of static hindlimb muscle stretch before (Control) and after ASIC1a blockade in SHAM (n = 6) and HF-rEF (n = 7) rats. Data were analysed with two-way ANOVA and Šidák’s multiple comparisons test and are expressed as means + SD. Asterisks indicate time points where comparisons were statistically significant (P < 0.05).
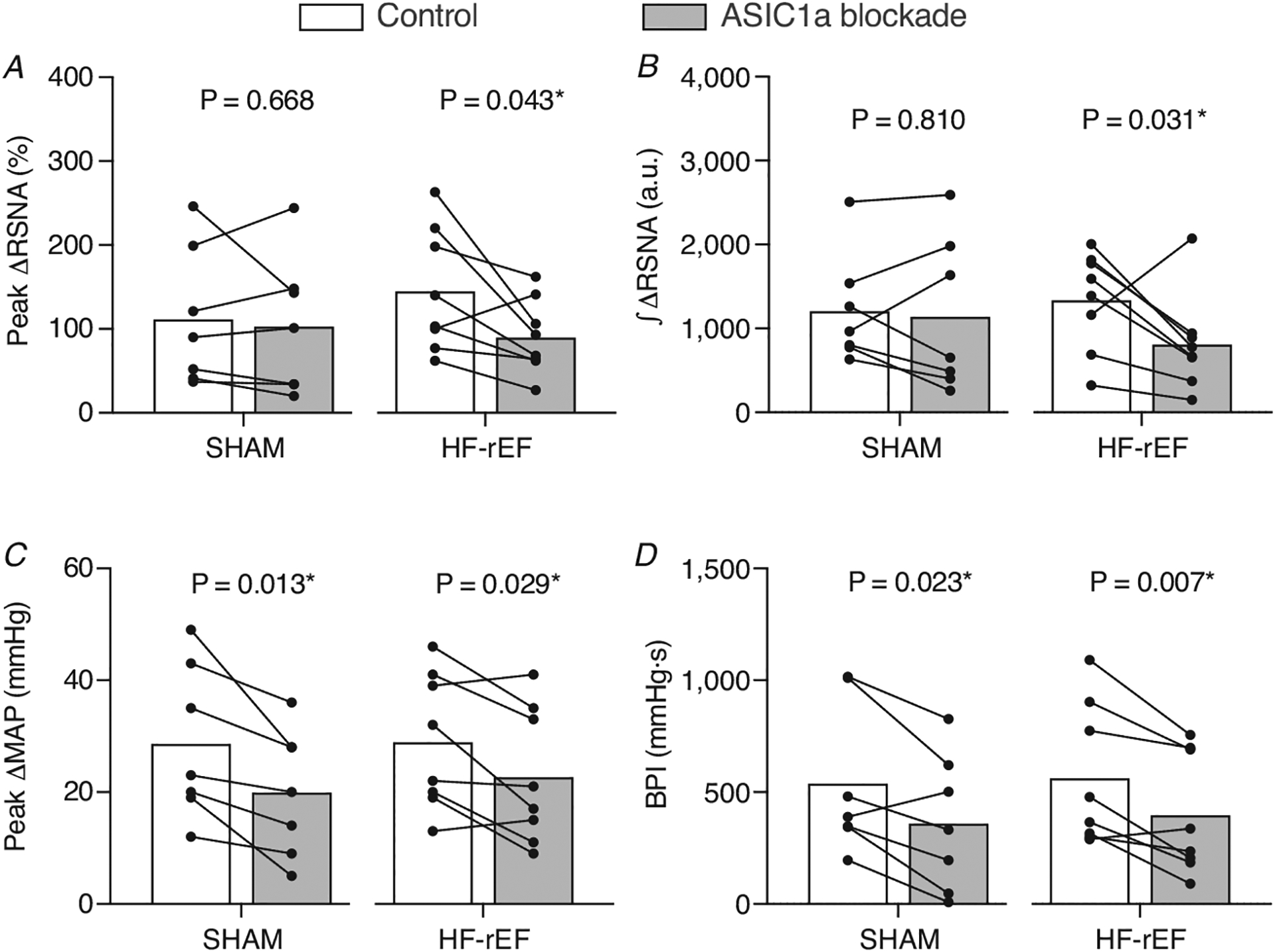
Figure 6. Effect of ASIC1a blockade on mechanoreflex activation.
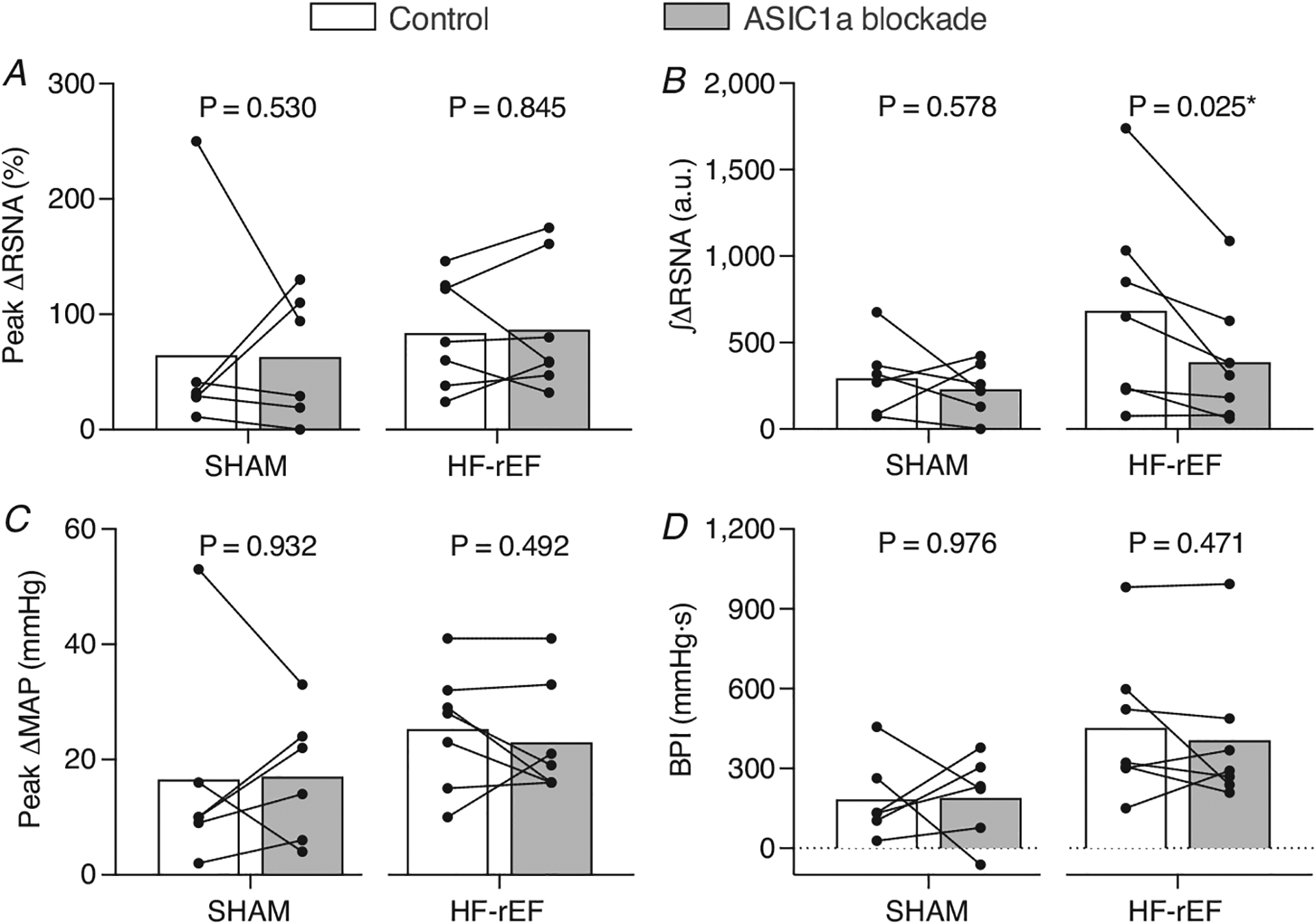
The peak Δ renal sympathetic nerve activity (RSNA; A) and integrated change in RSNA (∫ΔRSNA, B) as well as the peak Δ mean arterial pressure (MAP; C) and blood pressure index (BPI, D) in response to 30 s of static hindlimb muscle stretch before (Control) and after ASIC1a blockade in SHAM (n = 6) and HF-rEF (n = 7) rats. Data were analysed with multiple t-tests and are expressed as mean values overlaid with individual responses. Asterisks indicate statistically significant differences between conditions (P < 0.05).
Western blot and quantitative reverse transcriptase polymerase chain reaction experiments for ASIC1a/ASIC1 expression
In 36 rats (16 SHAM, 20 HF-rEF) the left and right L4 and L5 DRG were harvested. Samples were isolated into 2 ml bead mill tubes containing ~0.5 g of 1.4 mm ceramic beads and 300 μl of ML lysis buffer (Macherey-Nagel, Düren, Germany) and homogenized for 1 min at 5 m/s using Bead Mill 4 (Fisherbrand, Waltham, MA, USA). Total protein and mRNA from tissues were prepared with the Nucleospin miRNA/Protein Kit (Macherey-Nagel; ref. no. 740971.50) according to the manufacturer’s instructions. Total Protein and RNA concentrations were determined using the Qubit 2.0 Fluorometer (Thermo Fisher Scientific, Waltham, MA, USA). Protein samples (40 μg) were separated on 4–12% Bis-Tris Protein Gels (Thermo Fisher Scientific) by gel electrophoresis in MES running buffer (Thermo Fisher Scientific) employing 220 V for 22 min. Gels were then transferred to mini-polyvinylidene difluoride membranes using the iBlot 2 Dry Transfer Device (Thermo Fisher Scientific). The membrane was incubated for ~3 h with the iBind device with iBind solution (Thermo Fisher Scientific) with the primary antibodies: anti-ASIC1a diluted 1:100 (Santa Cruz Biotechnology, Dallas, TX, USA; cat. no. SC-515033; RRID: AB_2847878) and loading control antibody anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) diluted 1:1000 (Thermo Fisher Scientific; cat. no. MA5–15738; RRID: AB_10977387) as well as the secondary antibody conjugated with horseradish peroxidase diluted 1:1500 (Thermo Fisher Scientific; cat. no. 31430; RRID: AB_228307). Membranes were then incubated for 5 min with SuperSignal West Pico PLUS Chemiluminescent Substrate (Thermo Fisher Scientific) and imaged with C–DiGit Blot Scanner (Li-Cor Biosciences, Lincoln, NE, USA). The protein bands were quantified and analysed using the Image Studio software (Li-Cor). Complementary DNA (cDNA) was synthesized from RNA isolates (see above) using the High Capacity RNA-cDNA kit (Thermo Fisher Scientific) according to the manufacturer’s instructions as described previously (Copp et al., 2016). Quantitative reverse transcriptase polymerase chain reaction experiments were then performed on the cDNA samples using TaqMan gene expression assays specific for: ASIC1a (sequence proprietary; Assay ID: Rn01638093_g1), and GAPDH with forward primer: 5′-ACCGCCTGTTGCGTGTTA-3′ and reverse primer: 5′-CAATCGCCAACGCCTCAA-3′. All samples were run in duplicate for the gene of interest, and the endogenous control (GAPDH). The results were analysed with the comparative threshold (ΔΔCt) method. A technical difficulty prevented us from determining GAPDH mRNA expression in one HF-rEF rat, which resulted in 19 HF-rEF rats for the ASIC1/GAPDH mRNA expression analysis.
Data analysis
Muscle tension, blood pressure, HR and RSNA were measured and recorded in real time with a PowerLab and LabChart data acquisition system (ADInstruments). The original RSNA data were rectified and corrected for the background noise determined after the administration of hexamethonium bromide. Baselines for mean arterial pressure (MAP), RSNA and HR were determined from the 30 s baseline periods that preceded each manoeuvre. The peak increases in MAP (peak ΔMAP), RSNA (peak ΔRSNA) and HR (peak ΔHR) were calculated as the difference between the peak values wherever they occurred during the manoeuvres and their corresponding baseline value. The integrated RSNA (∫ΔRSNA) was calculated by integrating the ΔRSNA (>0) during the manoeuvre. The change in tension–time index (ΔTTI) and blood pressure index (BPI) were calculated by integrating the area under curve during the manoeuvre and subtracting the integrated area under the curve during the baseline period. Time courses of the increase in RSNA, MAP, HR and tension were plotted as their change from baseline and were analysed with two-way ANOVAs with Šidák’s multiple comparisons test. Multiple Student’s t-tests were performed for within-animal comparisons of baseline MAP, baseline HR, peak ΔMAP, peak ΔHR, peak ΔRSNA, BPI, ∫ΔRSNA and ΔTTI. One-way ANOVA with Šidák’s multiple comparison test was used to determine the effect of sequential blockades of μ- and δ-opioid receptor as well as ASIC1a on baseline MAP, baseline HR, peak ΔMAP, peak ΔHR, peak ΔRSNA, BPI, ∫ΔRSNA and ΔTTI in HF-rEF rats pre-treated with naloxone. Data for echocardiograph measurements, body mass, heart morphometrics, and protein and mRNA expression were analysed with an unpaired Student’s t-test or the Mann-Whitney U-test as appropriate. Pearson’s correlation coefficient and linear regression analyses were used to examine the relationship between ASIC1a protein expression and ejection fraction, fractional shortening, and left ventricular infarct size. Statistical significance was defined as P < 0.05. n values indicate total number of rats. Data are presented as means (SD).
Results
Body mass, heart morphometrics
Body mass as well as the ratio of LV to body mass were not different between SHAM and HF-rEF rats (Table 1). The ratios of the RV and atria mass to body mass were greater in HF-rEF rats compared to SHAM rats. Additionally, LVEDV and LVESV were significantly greater, and ejection fraction and fractional shortening were significantly lower, in HF-rEF rats compared to SHAM rats. There was no difference in SV between groups.
Table 1.
Body and tissue masses and heart morphometrics in SHAM and HF-rEF rats
| SHAM (n = 43) | HF-rEF (n = 59) | P | |
|---|---|---|---|
| Body mass (g) | 529 (45) | 525 (36) | 0.632 |
| LV/body mass (mg/g) | 1.97 (0.34) | 2.03 (0.42) | 0.228 |
| RV/body mass (mg/g) | 0.54 (0.06) | 0.57 (0.11) | 0.034* |
| Atria/body mass (mg/g) | 0.16 (0.04) | 0.22 (0.08) | <0.001* |
| LV EDV (ml) | 1.23 (0.29) | 2.10 (0.55) | <0.001* |
| LV ESV (ml) | 0.25 (0.15) | 1.14 (0.51) | <0.001* |
| Stroke volume (ml) | 0.99 (0.20) | 1.01 (0.24) | 0.363 |
| Ejection fraction (%) | 82 (7) | 45 (18) | <0.001* |
| Fractional shortening (%) | 46 (8) | 21 (10) | <0.001* |
| Infarct size (%) | — | 29 (9) | — |
Values are means (SD). EDV, end diastolic volume; ESV, end systolic volume; LV, left ventricle; RV, right ventricle. Data were compared using Student’s t-test.
Asterisks indicate statistical significance (P < 0.05).
Effect of ASIC1a blockade on reflex responses to lactic acid injections
Injection of PcTx-1 into the arterial supply of the hindlimb reduced the renal sympathetic and pressor response to injection of lactic acid into the arterial supply of the hindlimb in both SHAM (n = 12) and HF-rEF rats (n = 11; Fig. 1). Baseline MAP and HR before and after ASIC1a blockade for SHAM and HF-rEF rats are shown in Tables 2 and 3. These data provide evidence that injection of 100 ng of PcTx-1 into the arterial supply of the hindlimb effectively blocked ASIC1a on the sensory endings of thin fibre muscle afferents (referred to as ASIC1a blockade from this point forward for simplicity). Additionally, the peak ΔRSNA (SHAM: 71 (68)%; HF-rEF: 100 (50)%; P = 0.259) and peak ΔMAP (SHAM: 19 (12) mmHg; HF-rEF: 26 (21) mmHg; P = 0.347) response to lactic acid in the control conditions from these rats were not different between SHAM and HF-rEF rats.
Figure 1. Effect of ASIC1a blockade on renal sympathetic and pressor response to lactic acid injection.
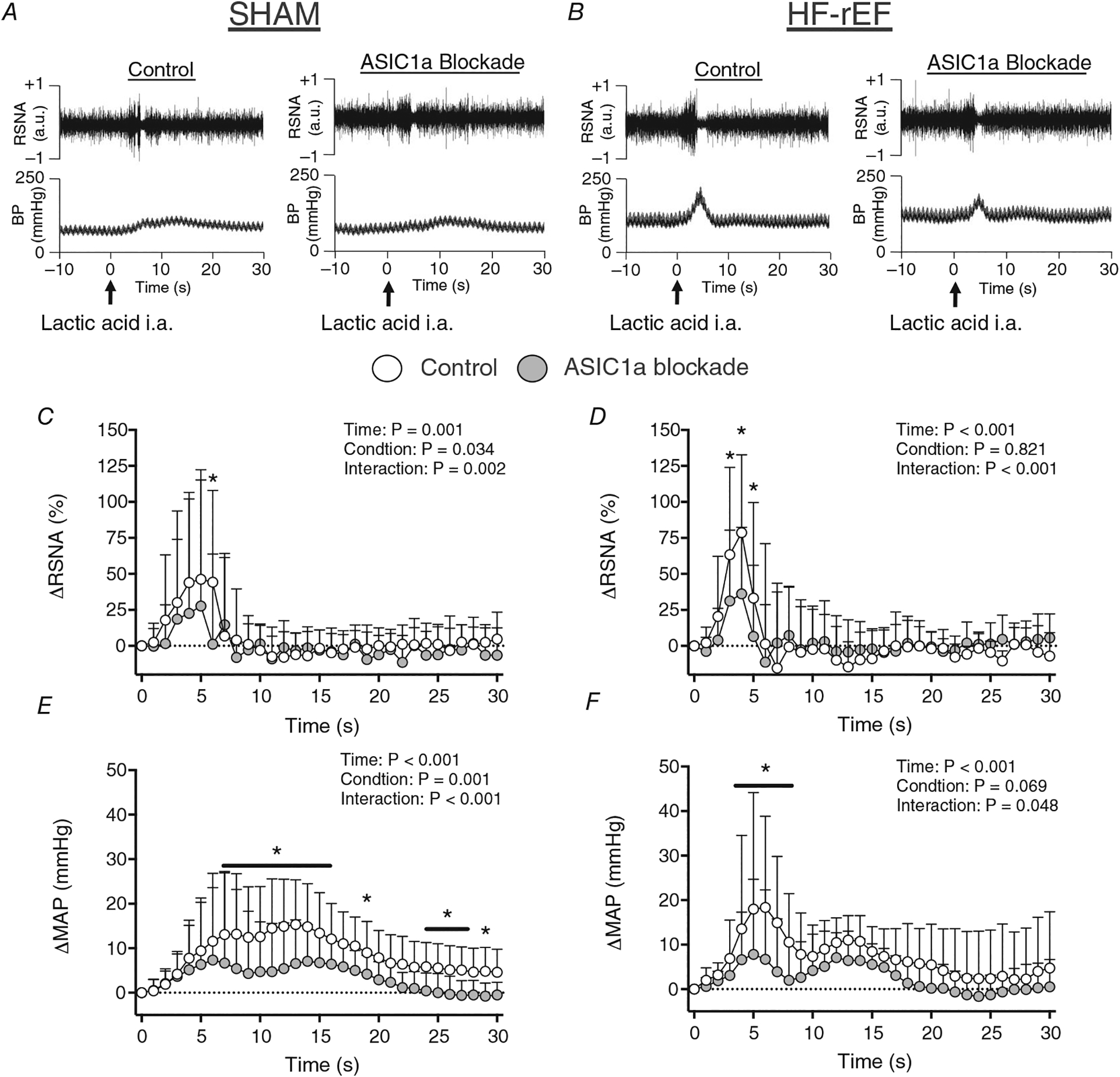
A and B, examples of original tracings of RSNA and blood pressure (BP) in response to lactic acid injection (indicated by arrows) before and after ASIC1a blockade in a SHAM (A) and HF-rEF (B) rat. C–F, Δ renal sympathetic nerve activity (RSNA; C and D) and Δ mean arterial pressure (MAP; E and F) in response to hindlimb arterial injection of lactic acid (24 mmol) before (Control) and after ASIC1a blockade in SHAM (n = 12) and HF-rEF (n = 11) rats. Data were analysed with two-way ANOVA and Šidák’s multiple comparisons test and are expressed as means + SD. Asterisks and/or black lines indicate time points where comparisons were statistically significant (P < 0.05).
Table 2.
Baseline MAP in SHAM and HF-rEF rats
| Experimental group | Method of PcTx-1 injection | MAP (mmHg) | P | |
|---|---|---|---|---|
| Control | Post-injection | |||
| SHAM lactic acid injection, n = 12 | i.a. | 76 (10) | 78 (12) | 0.514 |
| HF-rEF lactic acid injection, n = 11 | i.a. | 92 (25) | 93 (24) | 0.434 |
| SHAM contraction, n = 7 | i.a. | 88 (8) | 90 (14) | 0.678 |
| HF-rEF contraction, n = 8 | i.a. | 75 (12) | 75 (9) | 0.945 |
| SHAM contraction, n = 5 | i.v. | 74 (5) | 72 (6) | 0.535 |
| HF-rEF contraction, n = 5 | i.v. | 79 (16) | 77 (16) | 0.726 |
| SHAM stretch, n = 6 | i.a. | 93 (22) | 100 (16) | 0.300 |
| HF-rEF stretch, n = 7 | i.a. | 79 (12) | 88 (13) | 0.048* |
Values are means (SD). Experimental group is identified by the primary experimental manoeuvre performed. Data were compared with paired Student’s t-test. I.A., intra-arterial; I.V., intravenous; HF-rEF, heart failure with reduced ejection fraction; MAP, mean arterial pressure; PcTx-1, psalmotoxin-1.
Asterisk indicates statistical significance (P < 0.05).
Table 3.
Baseline HR and cardioaccelerator responses in SHAM and HF-rEF rats
| Experimental group | Method of PcTx-1 injection | HR (bpm) | P | |
|---|---|---|---|---|
| Control | Post-injection | |||
| Baseline HR | ||||
| SHAM lactic acid injection, n = 12 | i.a. | 488 (25) | 481 (10) | 0.318 |
| HF-rEF lactic acid injection, n = 11 | i.a. | 488 (45) | 483 (53) | 0.455 |
| SHAM contraction, n = 7 | i.a. | 419 (62) | 442 (53) | 0.198 |
| HF-rEF contraction, n = 8 | i.a. | 406 (33) | 414 (40) | 0.041* |
| SHAM contraction, n = 5 | i.v. | 440 (42) | 425 (35) | 0.240 |
| HF-rEF contraction, n = 5 | i.v. | 416 (55) | 407 (24) | 0.035* |
| SHAM stretch, n = 6 | i.a. | 490 (34) | 493 (24) | 0.682 |
| HF-rEF stretch, n = 7 | i.a. | 468 (27) | 463 (39) | 0.534 |
| Peak ΔHR | ||||
| SHAM lactic acid injection, n = 12 | i.a. | 8 (7) | 7 (5) | 0.024* |
| HF-rEF lactic acid injection, n = 11 | i.a. | 10 (6) | 7 (5) | 0.057 |
| SHAM contraction, n = 7 | i.a. | 30 (7) | 20 (5) | 0.037* |
| HF-rEF contraction, n = 8 | i.a. | 37 (12) | 28 (10) | 0.024* |
| SHAM contraction, n = 5 | i.v. | 28 (7) | 24 (6) | 0.535 |
| HF-rEF contraction, n = 5 | i.v. | 26 (12) | 21 (14) | 0.138 |
| SHAM stretch, n = 6 | i.a. | 8 (5) | 7 (4) | 0.767 |
| HF-rEF stretch, n = 7 | i.a. | 16 (9) | 13 (8) | 0.229 |
Values are mean (SD). Experimental group is identified by the primary experimental manoeuvre performed. Data were compared with paired Student’s t-test. i.a., intra-arterial; i.v., intravenous; HF-rEF, heart failure with reduced ejection fraction; HR, heart rate; PcTx-1, psalmotoxin-1.
Asterisks indicate statistical significance (P < 0.05).
Effect of ASIC1a blockade on the exercise pressor reflex
In SHAM rats (n = 7), ASIC1a blockade reduced the pressor and cardioaccelerator response to static hindlimb muscle contraction (Figs 2–4, and Table 3). However, ASIC1a blockade had no effect on the renal sympathetic response to contraction in SHAM rats. In HF-rEF rats (n = 8), ASIC1a blockade significantly reduced the RSNA, pressor and cardioaccelerator response to contraction (Figs 2–4, and Table 3). There was no difference between SHAM and HF-rEF rats in the magnitude of the effect of ASIC1a blockade on the BPI response (SHAM: −179 (156) mmHg s; HF-rEF: −166 (126) mmHg s; P = 0.858) or peak MAP response (SHAM: −9 (7) mmHg; HF-rEF: −6 (6) mmHg; P = 0.475) to contraction. The tension developed during the contraction manoeuvre was not different between control and ASIC1a blockade conditions in either group of rats (Fig. 2G and H). Baseline MAP and HR before and after ASIC1a blockade for SHAM and HF-rEF rats are shown in Tables 2 and 3, respectively. In vehicle control experiments, injection of 0.1 ml saline into the hindlimb arterial supply had no effect (P-value range: 0.124–0.775) on the RSNA, pressor or cardioaccelerator response to hindlimb muscle contraction in SHAM (n = 3) or HF-rEF (n = 4) rats (data not shown).
Figure 2. Effect of ASIC1a blockade on the time course of exercise pressor reflex activation.
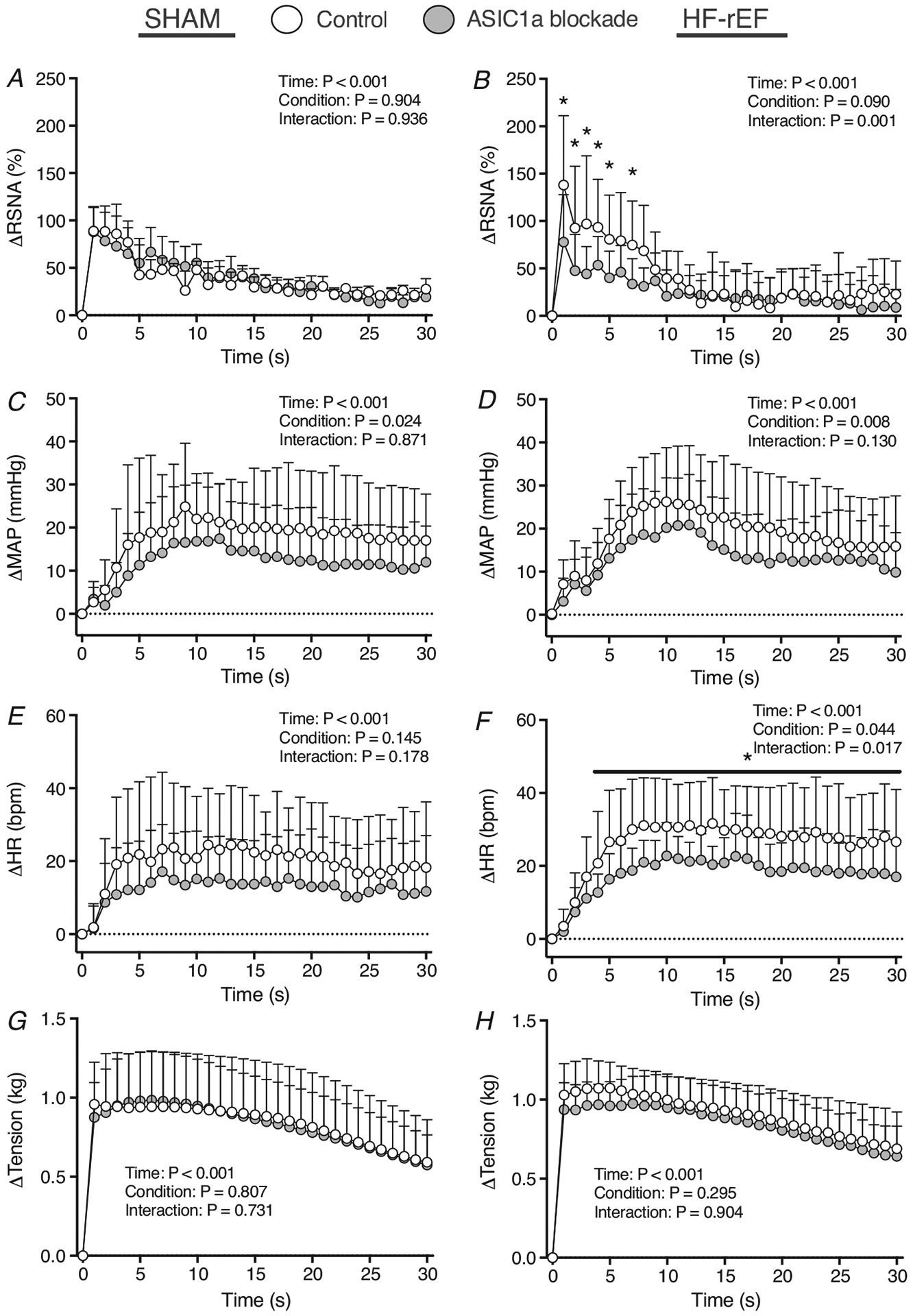
Δ renal sympathetic nerve activity (RSNA; A and B), Δ mean arterial pressure (MAP; C and D), Δ heart rate (HR; E and F), Δ and tension (G and H) in response to 30 s of static hindlimb muscle contraction before (Control) and after ASIC1a blockade in SHAM (n = 7) and HF-rEF (n = 8) rats. Data were analysed with two-way ANOVA and Šidák’s multiple comparisons test and are expressed as means + SD. Asterisks and/or black lines indicate time points where comparisons were statistically significant (P < 0.05).
Figure 4.
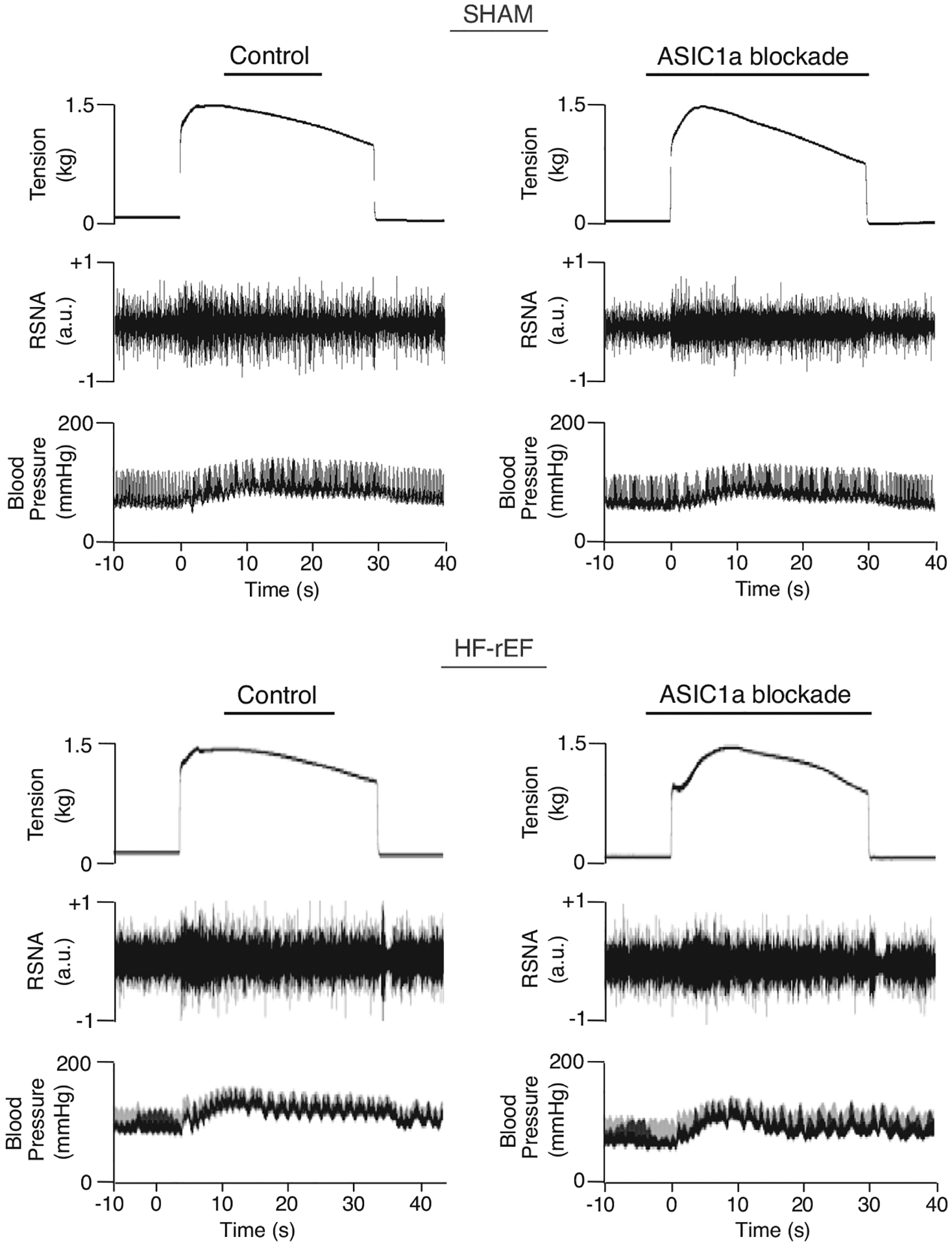
Examples of original tracings of renal sympathetic nerve activity (RSNA) and blood pressure evoked during 30 s of static hindlimb muscle contraction before (Control) and after ASIC1a blockade in a SHAM (left) and HF-rEF (right) rat.
In separate groups of SHAM (n = 5) and HF-rEF rats (n = 5), we investigated the possibility that systemic circulation of PcTx-1 accounted for the effect of the drug when it was injected into the arterial supply of the hindlimb. In SHAM rats, injection of PcTx1 into the jugular vein had no effect on the peak pressor (control: 22 (4) mmHg; PcTx-1: 18 (7) mmHg; P = 0.362), BPI (control: 405 (47) mmHg s; PcTx-1: 313 (123) mmHg s; P = 0.205) or peak cardioaccelerator response (Table 4) to hindlimb muscle contraction. Additionally, injection of PcTx1 into the jugular vein had no effect on the peak renal sympathetic (control: 138 (113)%; PcTx-1: 162 (134)%; P = 0.208) or integrated renal sympathetic nerve response (control: 958 (690) a.u.; PcTx-1: 845 (289) a.u.; P = 0.824) to contraction in SHAM rats. Likewise, injection of PcTx1 via the jugular vein of HF-rEF rats had no effect on the peak pressor (control: 20 (8) mmHg; PcTx-1: 20 (13) mmHg; P > 0.999), BPI (control: 358 (199)mmHg s; PcTx-1: 334 (240) mmHg s; P = 0.717), or peak cardioaccelerator response (Table 3) to hindlimb muscle contraction. Additionally, injection of PcTx1 into the jugular vein had no effect on the peak renal sympathetic (control: 81 (84)%; PcTx-1: 53 (33)%; P = 0.560) or integrated renal sympathetic (control: 884 (760) a.u.; PcTx-1: 553 (325) a.u.; P = 0.435) response to hindlimb muscle contraction in HF-rEF rats. The TTI of the contraction manoeuvre was not different between the control and i.v. PcTx-1 conditions for either SHAM (control: 25 (8) kg s; PcTx-1: 25 (9) kg s; P > 0.999) or HF-rEF rats (control: 15 (6) kg s; PcTx-1: 16 (3) kg s; P = 0.721). Baseline MAP and HR before and after injection of ASIC1a into the jugular vein for SHAM and HF-rEF rats are shown in Tables 2 and 3, respectively. The results of these experiments are consistent with similar control experiments performed by Ducrocq et al. (2020b) and suggest that the effect of PcTx-1 when it was injected into the hindlimb arterial supply of SHAM and HF-rEF rats was not attributable to circulating/systemic effects.
Table 4.
Pressor, cardioaccelerator and peak renal sympathetic response to static hindlimb muscle stretch before (control), after μ- and δ-opioid receptor blockade (naloxone) and after ASIC1a blockade in HF-rEF rats (n = 5)
| Control | Naloxone | ASIC1a blockade | P | |
|---|---|---|---|---|
| Peak ΔMAP (mmHg) | 30 (17) | 31 (15) | 32 (24) | 0.975 |
| BPI (mmHg s) | 513 (309) | 444 (412) | 503 (197) | 0.478 |
| Baseline MAP (mmHg) | 102 (7) | 103 (11) | 95 (16) | 0.361 |
| Peak ΔHR (bpm) | 13 (7) | 10 (7) | 12 (11) | 0.316 |
| Baseline HR (bpm) | 471 (48) | 470 (40) | 469 (40) | 0.904 |
| Peak ΔRSNA (%) | 128 (152) | 132 (81) | 112 (89) | 0.777 |
Values are means (SD). Data were compared with one-way ANOVA. BPI, blood pressure index; HF-rEF, heart failure with reduced ejection fraction; HR, heart rate; MAP, mean arterial pressure; RSNA, renal sympathetic nerve activity.
Effect of ASIC1a blockade on the mechanoreflex
In SHAM rats (n = 6), ASIC1a blockade had no effect on the RSNA, pressor or cardioaccelerator response to static hindlimb muscle stretch (Figs 5, 6 and Table 3). In HF-rEF rats (n = 7), ASIC1a blockade had no effect on the pressor or cardioaccelerator responses to stretch, but significantly reduced the RSNA response (Fig. 6D) especially within the first ~10 s of the stretch manoeuvre (Fig. 5B). The tension developed during the stretch manoeuvre was not different between control and ASIC1a blockade conditions at any point in either group of rats (Fig. 5G and H). Baseline MAP and HR before and after ASIC1a blockade for SHAM and HF-rEF rats are shown in Tables 2 and 3, respectively.
In a separate group of HF-rEF rats (n = 5), we investigated the possibility that the effect of ASIC1a blockade with PcTx-1 on the integrated renal sympathetic response to static hindlimb muscle stretch was attributed to a release of Met-enkephalin, a μ- and δ-opioid receptor agonist (Mazzuca et al., 2007). We found that pre-treating HF-rEF rats with naloxone (50 μg; 0.1 ml saline), a μ- and δ-opioid receptor antagonist, did not prevent the effect of ASIC1a blockade on the integrated renal sympathetic response to stretch (Fig. 7). Additionally, the pressor and cardioaccelerator response to stretch was not affected by naloxone or ASIC1a blockade in these HF-rEF rats (Table 4).
Figure 7. Effect of μ- and δ-opioid receptor blockade and ASIC1a blockade on mechanoreflex activation in HF-rEF rats.
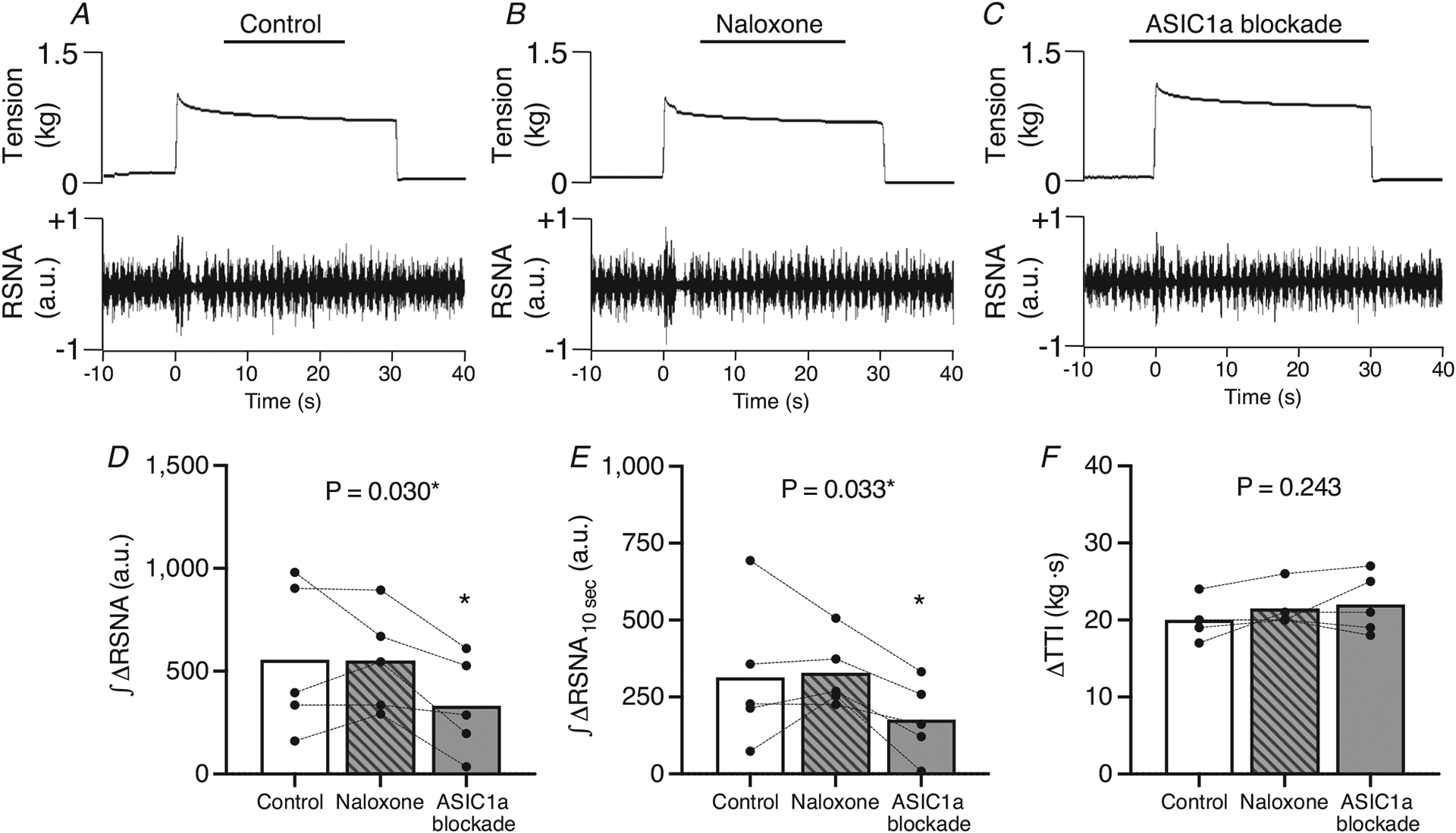
A–C, example of original tracings of the RSNA responses to 30 s of static hindlimb muscle stretch in control (A), post μ- and δ-opioid blockade (B), and post-ASIC1a blockade conditions (C) from a HF-rEF rat. D and E, the integrated change in renal sympathetic nerve activity (∫ΔRSNA, D) and the first 10 s of the integrated change in RSNA (∫ΔRSNA10s, E) in response to 30 s of static hindlimb muscle stretch before (Control) and after μ- and δ-opioid receptor blockade (naloxone) as well as after ASIC1a blockade in HF-rEF rats (n = 5). F, tension–time index (TTI). Data were analysed with one-way ANOVA and Šidák’s multiple comparisons test and are expressed as mean values overlaid with individual responses. Asterisks indicate P < 0.05 for ASIC1a blockade vs. control and μ- and δ-opioid blockade with Šidák’s multiple comparisons.
DRG ASIC1a protein and ASIC1 mRNA expression
There was no difference between SHAM (n = 16) and HF-rEF (n = 20) rats in ASIC1a protein or mRNA expression within L4 and L5 DRG tissue (Fig. 8). Interestingly, HF-rEF rats displayed a greater variance in ASIC1a protein expression (F = 9.01; P < 0.001), but not mRNA expression (F = 1.70; P = 0.306) within L4 and L5 DRG tissue. Moreover, in HF-rEF rats there was no significant linear relationship between ASIC1a protein expression and ejection fraction (P = 0.243, r = −0.274), fractional shortening (P = 0.340, r = −0.225), or left ventricular infarct size (P = 0.149, r = 0.335).
Figure 8. Effect of HF-rEF on ASIC1a expression.
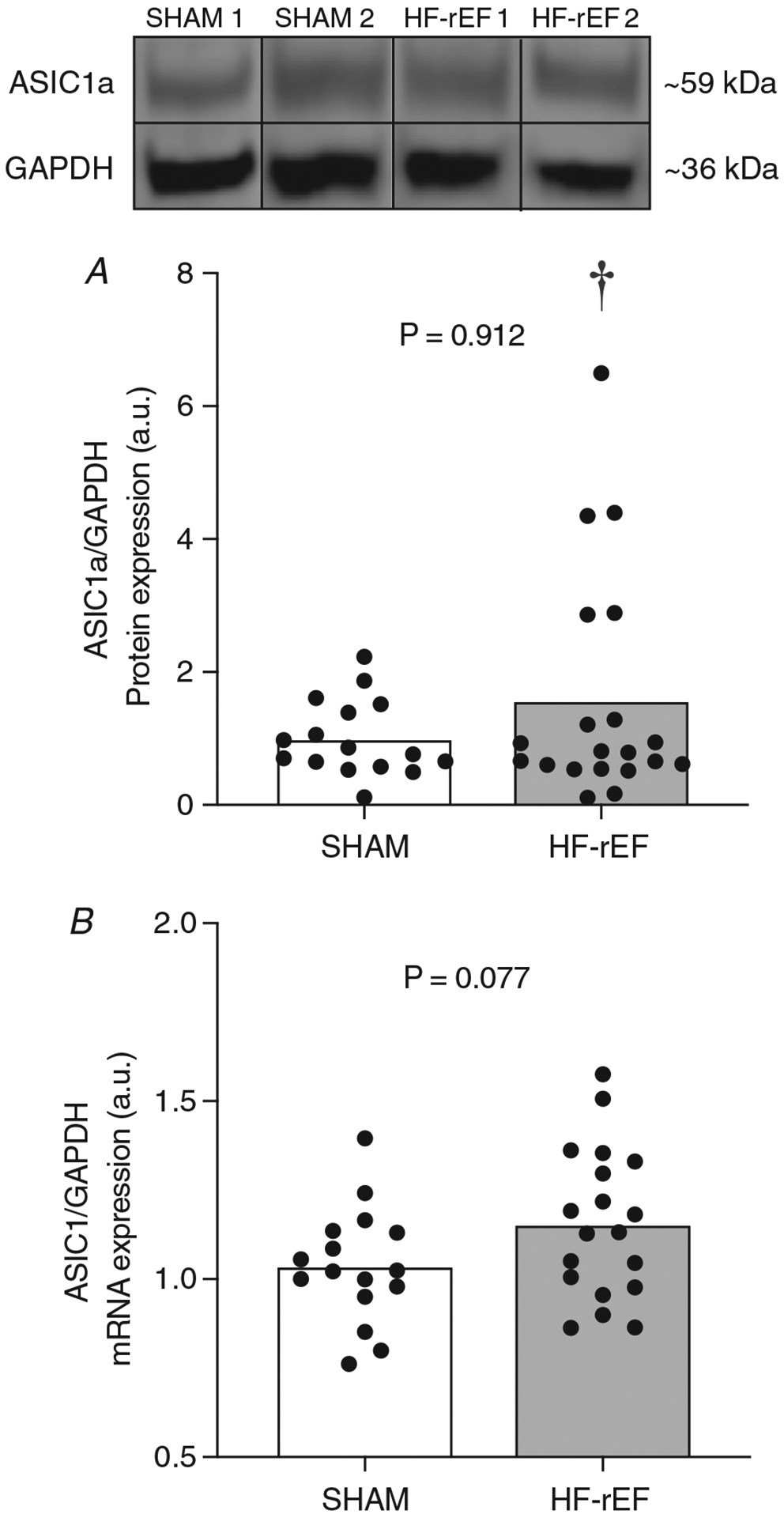
ASIC1a protein (A) and ASIC1 mRNA (B) in L4 and L5 dorsal root ganglia (DRG) tissue from SHAM (n = 16) and HF-rEF (n = 20) rats. GAPDH was used as a loading control (A) and reference sample (B). Examples of original western blot images from two sham and two HF-rEF rats are also shown. Data were analysed with a Mann–Whitney test (A) or Student’s t-test (B) and expressed as mean values overlaid with individual responses. †Statistically significant difference in variance between groups (P < 0.05).
Discussion
We investigated the role played by ASIC1a in evoking the exercise pressor reflex in a rat model of HF-rEF and in SHAM-operated healthy counterparts. We found that injecting the ASIC1a antagonist PcTx-1 into the arterial supply of the hindlimb attenuated the pressor and cardioaccelerator response to static hindlimb muscle contraction in SHAM and HF-rEF rats. We also found that ASIC1a blockade reduced the RSNA response to hindlimb muscle contraction in HF-rEF rats, but not in SHAM rats. Interestingly, in experiments investigating a possible role for ASIC1a in the mechanoreflex, we found that ASIC1a blockade reduced the RSNA response to hindlimb muscle stretch in HF-rEF rats but not in SHAM rats. Lastly, we found no difference between SHAM and HF-rEF rats in group mean ASIC1a protein or ASIC1 mRNA expression within L4/L5 DRG tissue, although HF-rEF rats did have significantly greater variance in ASIC1a protein expression. Collectively, the present results suggest that ASIC1a contributes to the activation of the exercise pressor reflex in both healthy rats and rats with HF-rEF. Moreover, the results indicate that, in HF-rEF rats, ASIC1a develops a role in mediating the mechanoreflex component of the exercise pressor reflex that is not present in healthy SHAM rats.
The tarantula toxin PcTx-1 is a highly selective and highly potent ASIC1a antagonist (IC50 of 1 nM; Escoubas et al., 2000). Ducrocq et al. (2020b) found that injection of ~100 ng of PcTx-1 into the arterial supply of a healthy rat hindlimb attenuated the reflex increase in blood pressure evoked in response to hindlimb arterial injection of lactic acid. We reproduced those findings in SHAM rats and extended them to HF-rEF rats (Fig. 1). In the control condition, we found no difference between SHAM and HF-rEF rats in the RSNA or pressor response to lactic acid injection. In contrast, Xing et al. (2015) previously reported a blunted pressor response to hindlimb arterial injection of lactic acid in HF-rEF rats compared to control rats. However, Xing et al. (2015) slowly infused lactic acid over 30 s, which likely stimulated ASIC3 to a greater extent than ASIC1a because ASIC1a currents are only generated when the pH is changed within 10 s (Alijevic et al., 2020) and because ASIC3 currents are sustained while ASIC1a currents are transient (Yagi et al., 2006). Conversely, we rapidly injected (less than ~2 s) lactic acid into the arterial supply of the hindlimb which likely resulted in a significantly greater stimulus to ASIC1a than did the method of lactic acid delivery utilized by Xing et al. (2015). This difference in delivery method and possible difference in degree of ASIC1a stimulation might explain why we did not find a blunted RSNA and pressor response to hindlimb arterial injection of lactic acid in HF-rEF rats compared to SHAM rats.
Our finding in SHAM rats that ASIC1a blockade reduced the pressor and cardioaccelerator response to static hindlimb muscle contraction is an important reproduction of the findings from Ducrocq et al. (2020a, 2020b). The fact that the MAP response to contraction appeared to be reduced by ASIC1a blockade generally throughout the 30 s manoeuvre but with the most clear effect occurring in the latter half of the contraction (see Fig. 2C), along with the fact that ASIC1a blockade had no effect on the reflex responses to hindlimb muscle stretch in SHAM rats, is consistent with the notion suggested by Ducrocq et al. (2020a, 2020b) that, in health, ASIC1a plays a role in the metaboreflex but not the mechanoreflex. A surprising result of the present investigation was that, despite finding ASIC1a-induced reductions in the pressor and cardioaccelerator response to contraction, we found no effect of ASIC1a blockade on the RSNA response to hindlimb muscle contraction in SHAM rats. One possibility for this apparent discrepancy is that ASIC1a stimulation contributes to reflex increases in SNA directed towards vascular beds/organs other than the kidneys. Another possibility is that ASIC1a plays only a minor/secondary role in mediating the reflex increase in RSNA and that redundant mechanisms preserved the RSNA response to muscle contraction following ASIC1a blockade in SHAM rats. Nevertheless, the present data further support the recent findings (Ducrocq et al., 2020a, 2020b) indicating that ASIC1a plays a role in evoking the exercise pressor reflex in healthy rats.
Our finding in HF-rEF rats that ASIC1a blockade reduced the RSNA, pressor and cardioaccelerator response to static hindlimb muscle contraction suggests that ASIC1a contributes importantly to the exercise pressor reflex in HF-rEF rats. Interestingly, the magnitude of effect of ASIC1a blockade on the pressor response was not different between SHAM and HF-rEF rats, which suggests that ASIC1a does not play a greater role evoking the exercise pressor reflex in HF-rEF rats compared to SHAM rats. However, in contrast to SHAM rats, in HF-rEF rats ASIC1a blockade reduced the RSNA response to hindlimb muscle contraction. Our finding that the RSNA response to hindlimb muscle contraction was reduced following ASIC1a blockade in HF-rEF rats, but not SHAM rats, suggests that activation of ASIC1a on the sensory endings of thin fibre muscle afferents may contribute to the exaggerated renal vasoconstriction during static handgrip exercise in HF-rEF patients compared with healthy controls (Middlekauff et al., 2000; Momen et al., 2004). The role for ASIC1a in the heightened RSNA response to exercise pressor reflex activation in HF-rEF rats may also have important implications for our understanding of the development of cardiorenal syndrome and consequent abnormal renal function which is frequently reported in HF-rEF patients (Ramchandra et al., 2019). Nevertheless, the effect of ASIC1a blockade on RSNA was evident in the first second of contraction and disappeared within ~10 s (see Fig. 2B). It seems unlikely that an accumulation of acidic by-products of muscle contraction and consequent stimulation of ASIC1a could account for the ASIC1a-mediated effect at the onset of muscle contraction, especially when considering data showing that the predominately metabolically sensitive group IV muscle afferents take ~5–30 s to be activated by static hindlimb muscle contraction (Kaufman et al., 1983, 1984; Wang, Li et al., 2010). An alternative explanation is that the ASIC1a blockade-induced reduction in the RSNA response to contraction in HF-rEF rats is attributable to a role for ASIC1a in the mechanoreflex. In support of this explanation, ASIC1a blockade reduced the RSNA response to hindlimb muscle stretch in HF-rEF rats; an effect that was consistent across two different HF-rEF groups with slight variations in the experimental protocols (see experimental considerations below for more discussion on the naloxone experiments). ASIC1a blockade in HF-rEF rats, however, had no effect on the pressor or cardioaccelerator response to hindlimb muscle stretch. We interpret these findings to suggest that the primary role for ASIC1a in evoking the exercise pressor reflex involves metaboreflex-related effects on MAP and HR. Additionally, in HF-rEF, ASIC1a develops a novel, secondary role in evoking the exercise pressor reflex that involves mechanoreflex-related effects on RSNA.
Our finding that ASIC1a contributes to the mechanoreflex in HF-rEF is consistent with a growing body of literature suggesting that ASIC1a stimulation may modulate the function of mechanically activated channels on the sensory endings of thin fibre muscle afferents under various physiological conditions (Chen & Wong, 2013; Gregory et al., 2018; Page et al., 2004; Ruan et al., 2021). For example, ASIC1a blockade with PcTx-1 has been shown to prevent the development of mechanical hyperalgesia following activity-induced pain in the mouse (Gregory et al., 2018). Additionally, ASIC1 knockout mice are resistant to the typical development of mechanical hyperalgesia following injection of the inflammation-inducing carrageenan solution into the gastrocnemius muscle (Walder et al., 2010). Passive hindlimb muscle stretch as performed in the present investigation activates a model of the mechanoreflex in the absence of any contraction-induced metabolic stimulus or change in local muscle venous effluent blood lactate concentration or pH (Stebbins et al., 1988). Thus, the results of the stretch experiments in the present investigation provide strong evidence that the role played by ASIC1a in mechanoreflex activation in HF-rEF is not related to its role in acid/pH sensing. Moreover, the present data suggest that the mechanosensory role for ASIC1a does not develop acutely during muscle contraction but rather is chronically, or persistently, present and then revealed when the mechanical stimulus of muscle stretch/contraction is present. The precise mechanism(s) by which ASIC1a plays a role in mechanosensation in HF-rEF is unknown (see recent review by Ruan et al., 2021). Whether a ‘direct’ role for ASICs in mechanosensation exists in specific physiological/pathological conditions through, for example, proteins linking ASICs to the cytoskeleton, and/or whether ASIC-mediated intracellular signalling modulates the function of other ‘inherently’ mechano-sensitive proteins such as PIEZO channels (Coste et al., 2010), requires further investigation.
To our knowledge, the present investigation is the first to examine the effect of HF-rEF on ASIC1a expression in DRG neurons. In contrast to our hypothesis, we found no difference in ASIC1 mRNA or ASIC1a group mean protein expression within L4/L5 DRG tissue between SHAM and HF-rEF rats. Interestingly, however, we found greater variance in ASIC1a protein expression in HF-rEF rats compared to SHAM rats. We did not find greater variance in ASIC1 mRNA expression in HF-rEF rats although there was a trend (P = 0.077) towards greater group mean ASIC1 mRNA expression in HF-rEF compared to SHAM rats. These findings suggest that increased ASIC1a protein expression on the sensory endings of thin fibre muscle afferents may contribute to the role played by ASIC1a in the exaggerated exercise pressor reflex in some, but likely not the majority of, HF-rEF rats. It is important to note, however, that any such increase in ASIC1a protein expression could not account, in and of itself, for the mechanoreflex-related role played by ASIC1a, a role that was found to be remarkably consistent in the HF-rEF rats. In support of this notion, Walder et al. (2010) reported that carrageenan-induced mouse gastrocnemius muscle inflammation produced ASIC1a-mediated mechanical hyperalgesia in the absence of increased ASIC1 mRNA expression in lumbar DRG tissue. Our current finding that ASIC1a adopts a role in the mechanoreflex in HF-rEF rats that is not present in healthy rats in the absence of group mean differences in ASIC1a expression is consistent with those findings of Walder et al. (2010)
Several experimental considerations should be noted. First, only male rats were used in the present investigation. Future studies are needed to determine the role of ASIC1a signalling in the exercise pressor reflex in female rats with and without HF-rEF. Second, ASIC1a is also located on vascular smooth muscle cells which, when activated, results in vasoconstriction (Drummond et al., 2017). Thus, the possibility exists that injection of PcTx-1 into the arterial supply of the hindlimb could accentuate the increases in hindlimb arterial blood flow thereby resulting in a diminished stimulus to the metaboreflex produced by contraction-induced metabolite production. However, Ducrocq et al. (2020b) addressed this possibility and showed that injection of PcTx-1 into the arterial supply of the rat hindlimb had no effect on blood flow to the hindlimb during static contraction of the triceps surae muscle (assessed by popliteal artery blood flow). Third, we recognized the possibility that ASIC1a blockade with PcTx-1 may have resulted in the release of Met-enkephalin (Mazzuca et al., 2007), a μ- and δ-opioid receptor agonist, which may have accounted for our finding in HF-rEF rats that ASIC1a blockade reduced the RSNA response to static hindlimb muscle stretch. However, we found that ASIC1a blockade reduced the RSNA response to static hindlimb muscle stretch in a separate group of HF-rEF rats that were pre-treated with naloxone (a μ- and δ-opioid receptor antagonist) suggesting that μ- and δ-opioid receptor activation does not account for the attenuating effects of ASIC1a blockade on the mechanoreflex in HF-rEF rats. Fourth, we investigated ASIC1 mRNA and ASIC1a protein expression in whole DRG tissue. Thus, the mRNA and protein signals we investigated did not originate solely from group III and IV muscle afferents. Future studies should seek to determine ASIC1 mRNA and ASIC1a protein expression in isolated group III and IV muscle afferents from SHAM and HF-rEF rats. Fifth, during whole body exercise, the exercise pressor reflex works in concert with various other autonomic control signals which contribute to the sympathetic and cardiovascular adjustments to exercise in health and disease. For example, central command (Koba et al., 2006), the carotid chemoreflex (Li et al., 2006; Machado et al., 2020; Stickland et al., 2007) and the arterial baroreflex (Mancia et al., 1992; Grassi et al., 1995) have all been reported to contribute to sympathetic and cardiovascular responses during exercise. The extent to which ASIC1a contributes to the sympathetic and cardiovascular adjustments to whole body exercise when all these autonomic control mechanisms are working together remains unknown. Lastly, we do not report a statistical comparison of the reflex responses to muscle contraction or stretch between SHAM and HF-rEF rats from the control condition (i.e. before ASIC1a blockade). The present study was powered only to investigate the effect of ASIC1a blockade within the SHAM and HF-rEF groups and the across-group comparisons were left underpowered. Importantly, the available literature consistently demonstrates that, compared to SHAM counterparts, the reflex responses to hindlimb muscle contraction and stretch are exaggerated in the rat myocardial infarction-induced model of HF-rEF (Butenas et al., 2020, 2021b, 2021c; Koba et al., 2008, 2009, 2010; Morales et al., 2012; Smith et al., 2003; Smith, Mitchell et al., 2005; Smith, Williams et al., 2005; Wang, Pan et al., 2010). We do report the statistical comparison of the pressor response to lactic acid injection between SHAM and HF-rEF groups because, to our knowledge, this is the first investigation to report the reflex pressor and RSNA response to a bolus injection of lactic acid into the arterial supply of the hindlimb of HF-rEF rats.
In summary, we found that ASIC1a contributed to activation of the exercise pressor reflex in SHAM and HF-rEF rats. The present findings further suggest that ASIC1a plays a role only in evoking the metaboreflex component of the exercise pressor reflex in health whereas in in HF-rEF, ASIC1a plays a role in evoking both the metaboreflex and mechanoreflex components of the exercise pressor reflex. Targeting ASIC1a in HF-rEF patients may serve as a favourable therapeutic strategy aimed at mitigating exercise intolerance and cardiovascular risk. That conclusion is supported by the findings of Smith et al. (2020) who demonstrated that exercise pressor reflex activation in HF-rEF patients constrains increases in stroke volume and elevates systemic vascular resistance during exercise.
Supplementary Material
Figure 3. Effect of ASIC1a blockade on exercise pressor reflex activation.
The peak Δ renal sympathetic nerve activity (RSNA; A) and integrated change in RSNA (∫ΔRSNA, B) as well as the peak Δ mean arterial pressure (MAP; C) and blood pressure index (BPI, D) in response to 30 s of static hindlimb muscle contraction before (Control) and after ASIC1a blockade in SHAM (n = 7) and HF-rEF (n = 8) rats. Data were analysed with multiple t-tests and are expressed as mean values overlaid with individual responses. Asterisks indicate statistically significant differences between groups (P < 0.05).
Key points.
Skeletal muscle contraction results in exaggerated reflex increases in sympathetic nerve activity in heart failure patients compared to healthy counterparts, which likely contributes to increased cardiovascular risk and impaired tolerance for even mild exercise (i.e. activities of daily living) for patients suffering with this condition.
Activation of acid sensing ion channel subtype 1a (ASIC1a) on the sensory endings of thin fibre muscle afferents during skeletal muscle contraction contributes to reflex increases in sympathetic nerve activity and blood pressure, at least in healthy subjects.
In this study, we demonstrate that ASIC1a on the sensory endings of thin fibre muscle afferents plays a role in both the mechanical and metabolic components of the exercise pressor reflex in male rats with heart failure.
The present data identify a novel role for ASIC1a in evoking the exercise pressor reflex in heart failure and may have important clinical implications for heart failure patients.
Funding
This work was supported by the National Institutes of Health (NIH) (R01HL142877 awarded to S.W.C. and F31HL154779 awarded to K.S.R.) as well as the American College of Sports Medicine Foundation Doctoral Student Research Grant awarded to A.L.E.B.
Biography

Alec L. E. Butenas is a PhD student in the Department of Kinesiology at Kansas State University and is mentored by Dr Steven W. Copp in the Autonomic Neurophysiology Laboratory. His research is focused on understanding the role played by thin fibre muscle afferents in the control of the sympathetic nervous system during exercise in health and disease.
Footnotes
Competing interests
No competing interests declared.
Supporting information
Additional supporting information can be found online in the Supporting Information section at the end of the HTML view of the article. Supporting information files available:
Peer Review History
Statistical Summary Document
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Abboud FM, & Benson CJ (2015). ASICs and cardiovascular homeostasis. Neuropharmacology, 94, 87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alijevic O, Bignucolo O, Hichri E, Peng Z, Kucera JP, & Kellenberger S (2020). Slowing of the time course of acidification decreases the acid-sensing ion channel 1a current amplitude and modulates action potential firing in neurons. Frontiers in Cellular Neuroscience, 14, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF, & Dempsey JA (2011). Implications of group III and IV muscle afferents for high-intensity endurance exercise performance in humans. Journal of Physiology, 589, 5299–5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Runnels S, Morgan DE, Trinity JD, Fjeldstad AS, Wray DW, Reese VR, & Richardson RS (2011). On the contribution of group III and IV muscle afferents to the circulatory response to rhythmic exercise in humans. Journal of Physiology, 589, 3855–3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Venturelli M, Ives SJ, Morgan DE, Gmelch B, Witman MA, Jonathan GH, Walter WD, Stehlik J, & Richardson RS (2014). Group III/IV muscle afferents impair limb blood in patients with chronic heart failure. International Journal of Cardiology, 174, 368–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansorge EJ, Augustyniak RA, Perinot ML, Hammond RL, Kim JK, Sala-Mercado JA, Rodriguez J, Rossi NF, & O’Leary DS (2004). Altered muscle metaboreflex control of coronary blood flow and ventricular function in heart failure. American Journal of Physiology. Heart and Circulatory Physiology, 288, H1381–H1388. [DOI] [PubMed] [Google Scholar]
- Arnolda L, Brosnan J, Rajagopalan B, & Radda GK (1991). Skeletal muscle metabolism in heart failure in rats. American Journal of Physiology, 261, H434–H442. [DOI] [PubMed] [Google Scholar]
- Baumfalk DR, Opoku-Acheampong AB, Caldwell JT, Butenas ALE, Horn AG, Kunkel ON, Copp SW, Ade CJ, Musch TI, & Behnke BJ (2021). Effects of high-intensity training on prostate cancer-induced cardiac atrophy. American Journal of Translational Research, 13, 197–209. [PMC free article] [PubMed] [Google Scholar]
- Butenas ALE, Colburn TD, Baumfalk DR, Ade CJ, Hageman KS, Copp SW, Poole DC, & Musch TI (2021a). Angiotensin converting enzyme inhibition improves cerebrovascular control during exercise in male rats with heart failure. Respiratory Physiology & Neurobiology, 286, 103613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butenas ALE, Rollins KS, Matney JE, Williams AC, Kleweno TE, Parr SK, Hammond ST, Ade CJ, Hageman KS, Musch TI, & Copp SW (2020). No effect of endoperoxide 4 or thromboxane A2 receptor blockade on static mechanoreflex activation in rats with heart failure. Experimental Physiology, 105, 1840–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butenas ALE, Rollins KS, Williams AC, Parr SK, Hammond ST, Ade CJ, Hageman KS, Musch TI, & Copp SW (2021b). Exaggerated sympathetic and cardiovascular responses to dynamic mechanoreflex activation in rats with heart failure: Role of endoperoxide 4 and thromboxane A2 receptors. Autonomic Neuroscience, 232, 102784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butenas ALE, Rollins KS, Williams AC, Parr SK, Hammond ST, Ade CJ, Hageman KS, Musch TI, & Copp SW (2021c). Thromboxane A2 receptors contribute to the exaggerated exercise pressor reflex in male rats with heart failure. Physiological Reports, 9, e15052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos MO, Mansur DE, Mattos JD, Paiva ACS, Videira RLR, Macefield VG, da Nobrega ACL, & Fernandes IA (2019). Acid-sensing ion channels blockade attenuates pressor and sympathetic responses to skeletal muscle metaboreflex activation in humans. Journal of Applied Physiology, 127, 1491–1501. [DOI] [PubMed] [Google Scholar]
- Chen CC, & Wong CW (2013). Neurosensory mechano-transduction through acid-sensing ion channels. Journal of Cellular and Molecular Medicine, 17, 337–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copp SW, Kim JS, Ruiz-Velasco V, & Kaufman MP (2016). The mechano-gated channel inhibitor GsMTx4 reduces the exercise pressor reflex in decerebrate rats. Journal of Physiology, 594, 641–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, Dubin AE, & Patapoutian A (2010). Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science, 330, 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig JC, Colburn TD, Hirai DM, Musch TI, & Poole DC (2019). Sexual dimorphism in the control of skeletal muscle interstitial PO2 of heart failure rats: effects of dietary nitrate supplementation. Journal of Applied Physiology, 126, 1184–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibas J, Al-Saad H, & Dibas A (2019). Basics on the use of acid-sensing ion channels’ inhibitors as therapeutics. Neural Regeneration Research, 14, 395–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond HA, Xiang L, Chade AR, & Hester R (2017). Enhanced maximal exercise capacity, vasodilation to electrical muscle contraction, and hind limb vascular density in ASIC1a null mice. Physiological Reports, 5, e13368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducrocq GP, Kim JS, Estrada JA, & Kaufman MP (2020a). ASIC1a does not play a role in evoking the metabolic component of the exercise pressor reflex in a rat model of peripheral artery disease. American Journal of Physiology. Heart and Circulatory Physiology 319, H171–H182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducrocq GP, Kim JS, Estrada JA, & Kaufman MP (2020b). ASIC1a plays a key role in evoking the metabolic component of the exercise pressor reflex in rats. American Journal of Physiology. Heart and Circulatory Physiology, 318, H78–H89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escoubas P, De Weille JR, Lecoq A, Diochot S, Waldmann R, Champigny G, Moinier D, Menez A, & Lazdunski M (2000). Isolation of a tarantula toxin specific for a class of proton-gated Na+ channels. Journal of Biological Chemistry, 275, 25116–25121. [DOI] [PubMed] [Google Scholar]
- Gautam M, & Benson CJ (2013). Acid-sensing ion channels (ASICs) in mouse skeletal muscle afferents are heteromers composed of ASIC1a, ASIC2, and ASIC3 subunits. FASEB Journal, 27, 793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi G, Seravalle G, Cattaneo BM, Lanfranchi A, Vailati S, Giannattasio C, Del Bo A, Sala C, Bolla GB, & Pozzi M (1995). Sympathetic activation and loss of reflex sympathetic control in mild congestive heart failure. Circulation, 92, 3206–3211. [DOI] [PubMed] [Google Scholar]
- Gregory NS, Gautam M, Benson CJ, & Sluka KA (2018). Acid sensing ion channel 1a (ASIC1a) mediates activity-induced pain by modulation of heteromeric ASIC channel kinetics. Neuroscience, 386, 166–174. [DOI] [PubMed] [Google Scholar]
- Hammond RL, Augustyniak RA, Rossi NF, Churchill PC, Lapanowski K, & O’Leary DS (2000). Heart failure alters the strength and mechanisms of the muscle metaboreflex. American Journal of Physiology. Heart and Circulatory Physiology, 278, H818–H828. [DOI] [PubMed] [Google Scholar]
- Hayes SG, Kindig AE, & Kaufman MP (2007). Blockade of acid sensing ion channels attenuates the exercise pressor reflex in cats. Journal of Physiology, 581, 1271–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselager M, Timmermann DB, & Ahring PK (2004). pH dependency and desensitization kinetics of heterologously expressed combinations of acid-sensing ion channel subunits. Journal of Biological Chemistry, 279, 11006–11015. [DOI] [PubMed] [Google Scholar]
- Kaufman MP, & Forster HV (1996). Reflexes controlling circulatory, ventilatory and airway responses to exercise. In Rowell LB & Shepherd JT (eds.), Handbook of physiology, section 12: Exercise: Regulation and integration of multiple systems, II Control of respiratory and cardiovascular systems (pp. 381–447). Oxford University Press, New York. [Google Scholar]
- Kaufman MP, Iwamoto GA, Longhurst JC, & Mitchell JH (1982). Effects of capsaicin and bradykinin on afferent fibers with endings in skeletal muscle. Circulation Research, 50, 133–139. [DOI] [PubMed] [Google Scholar]
- Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH, & Mitchell JH (1983). Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. Journal of Applied Physiology, 55, 105–112. [DOI] [PubMed] [Google Scholar]
- Kaufman MP, Rybicki KJ, Waldrop TG, & Ordway GA (1984). Effect of ischemia on responses of group III and IV afferents to contraction. Journal of Applied Physiology, 57, 644–650. [DOI] [PubMed] [Google Scholar]
- Kempf EA, Rollins KS, Hopkins TD, Butenas AL, Santin JM, Smith JR, & Copp SW (2018). Chronic femoral artery ligation exaggerates the pressor and sympathetic nerve responses during dynamic skeletal muscle stretch in decerebrate rats. American Journal of Physiology. Heart and Circulatory Physiology, 314, H246–H254. [DOI] [PubMed] [Google Scholar]
- Kim JS, Ducrocq GP, & Kaufman MP (2020). Functional knockout of ASIC3 attenuates the exercise pressor reflex in decerebrated rats with ligated femoral arteries. American Journal of Physiology. Heart and Circulatory Physiology, 318, H1316–H1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JS, Harms JE, Ruiz-Velasco V, & Kaufman MP (2019). Effect of knockout of the ASIC3 on cardiovascular reflexes arising from hindlimb muscle in decerebrated rats. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 317, R641–R648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koba S, Gao Z, & Sinoway LI (2009). Oxidative stress and the muscle reflex in heart failure. Journal of Physiology, 587, 5227–5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koba S, Gao Z, Xing J, Sinoway LI, & Li J (2006). Sympathetic responses to exercise in myocardial infarction rats: a role of central command. American Journal of Physiology. Heart and Circulatory Physiology, 291, H2735–H2742. [DOI] [PubMed] [Google Scholar]
- Koba S, Xing J, Sinoway LI, & Li J (2008). Sympathetic nerve responses to muscle contraction and stretch in ischemic heart failure. American Journal of Physiology. Heart and Circulatory Physiology, 294, H311–H321. [DOI] [PubMed] [Google Scholar]
- Koba S, Xing J, Sinoway LI, & Li J (2010). Bradykinin receptor blockade reduces sympathetic nerve response to muscle contraction in rats with ischemic heart failure. American Journal of Physiology. Heart and Circulatory Physiology, 298, H1438–H1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolettis TM, Agelaki MG, Baltogiannis GG, Vlahos AP, Mourouzis I, Fotopoulos A, & Pantos C (2007). Comparative effects of acute vs. chronic oral amiodarone treatment during acute myocardial infarction in rats. Europace, 9, 1099–1104. [DOI] [PubMed] [Google Scholar]
- Li YL, Xia XH, Zheng H, Gao L, Li YF, Liu D, Patel KP, Wang W, & Schultz HD (2006). Angiotensin II enhances carotid body chemoreflex control of sympathetic outflow in chronic heart failure rabbits. Cardiovascular Research, 71, 129–138. [DOI] [PubMed] [Google Scholar]
- Machado AC, Vianna LC, Gomes EAC, Teixeira JAC, Ribeiro ML, Villacorta H, Nobrega ACL, & Silva BM (2020). Carotid chemoreflex and muscle metaboreflex interact to the regulation of ventilation in patients with heart failure with reduced ejection fraction. Physiological Reports, 8, e14361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancia G, Seravalle G, Giannattasio C, Bossi M, Preti L, Cattaneo BM, & Grassi G (1992). Reflex cardiovascular control in congestive heart failure. The American Journal of Cardiology, 69, 17–23. Discussion 22G–23G. [DOI] [PubMed] [Google Scholar]
- Massie BM, Conway M, Rajagopalan B, Yonge R, Frostick S, Ledingham J, Sleight P, & Radda G (1988). Skeletal muscle metabolism during exercise under ischemic conditions in congestive heart failure. Evidence for abnormalities unrelated to blood flow. Circulation, 78, 320–326. [DOI] [PubMed] [Google Scholar]
- Mazzuca M, Heurteaux C, Alloui A, Diochot S, Baron A, Voilley N, Blondeau N, Escoubas P, Gelot A, Cupo A, Zimmer A, Zimmer AM, Eschalier A, & Lazdunski M (2007). A tarantula peptide against pain via ASIC1a channels and opioid mechanisms. Nature Neuroscience, 10, 943–945. [DOI] [PubMed] [Google Scholar]
- McCloskey DI, & Mitchell JH (1972). Reflex cardiovascular and respiratory responses originating in exercising muscle. Journal of Physiology, 224, 173–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord JL, Hayes SG, & Kaufman MP (2008). Acid-sensing ion and epithelial sodium channels do not contribute to the mechanoreceptor component of the exercise pressor reflex. American Journal of Physiology. Heart and Circulatory Physiology, 295, H1017–H1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord JL, Tsuchimochi H, & Kaufman MP (2009). Acid-sensing ion channels contribute to the metaboreceptor component of the exercise pressor reflex. American Journal of Physiology. Heart and Circulatory Physiology, 297, H443–H449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mense S, & Meyer H (1988). Bradykinin-induced modulation of the response behavour of different types of feline group III and IV muscle receptors. Journal of Physiology, 398, 49–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mense S, & Stahnke M (1983). Responses in muscle afferent fibers of slow conduction velocity to contractions and ischemia in the cat. Journal of Physiology, 342, 383–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middlekauff HR, Chiu J, Hamilton MA, Fonarow GC, Maclellan WR, Hage A, Moriguchi J, & Patel J (2004). Muscle mechanoreceptor sensitivity in heart failure. American Journal of Physiology. Heart and Circulatory Physiology, 287, H1937–H1943. [DOI] [PubMed] [Google Scholar]
- Middlekauff HR, Nitzsche EU, Hoh CK, Hamilton MA, Fonarow GC, Hage A, & Moriguchi JD (2000). Exaggerated renal vasoconstriction during exercise in heart failure patients. Circulation, 101, 784–789. [DOI] [PubMed] [Google Scholar]
- Mitchell JH, Kaufman MP, & Iwamoto GA (1983). The exercise pressor reflex: Its cardiovascular effects, afferent mechanisms, and central pathways. Annual Review of Physiology, 45, 229–242. [DOI] [PubMed] [Google Scholar]
- Momen A, Bower D, Boehmer J, Kunselman AR, Leuenberger UA, & Sinoway LI (2004). Renal blood flow in heart failure patients during exercise. American Journal of Physiology. Heart and Circulatory Physiology, 287, H2834–H2839. [DOI] [PubMed] [Google Scholar]
- Morales A, Gao W, Lu J, Xing J, & Li J (2012). Muscle cyclo-oxygenase-2 pathway contributes to the exaggerated muscle mechanoreflex in rats with congestive heart failure. Experimental Physiology, 97, 943–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy SP, Kakkar R, McCarthy CP, & Januzzi JL Jr (2020). Inflammation in heart failure: JACC State-of-the-art review. Journal of the American College of Cardiology, 75, 1324–1340. [DOI] [PubMed] [Google Scholar]
- Musch TI, & Terrell JA (1992). Skeletal muscle blood flow abnormalities in rats with a chronic myocardial infarction: rest and exercise. American Journal of Physiology, 262, H411–H419. [DOI] [PubMed] [Google Scholar]
- O’Leary DS, Augustyniak RA, Ansorge EJ, & Collins HL (1999). Muscle metaboreflex improves O2 delivery to ischemic active skeletal muscle. American Journal of Physiology, 276, H1399–H1403. [DOI] [PubMed] [Google Scholar]
- Page AJ, Brierley SM, Martin CM, Martinez-Salgado C, Wemmie JA, Brennan TJ, Symonds E, Omari T, Lewin GR, Welsh MJ, & Blackshaw LA (2004). The ion channel ASIC1 contributes to visceral but not cutaneous mechanoreceptor function. Gastroenterology, 127, 1739–1747. [DOI] [PubMed] [Google Scholar]
- Piepoli M, Clark AL, Volterrani M, Adamopoulos S, Sleight P, & Coats AJ (1996). Contribution of muscle afferents to the hemodynamic, autonomic, and ventilatory responses to exercise in patients with chronic heart failure: effects of physical training. Circulation, 93, 940–952. [DOI] [PubMed] [Google Scholar]
- Ramchandra R, Xing DT, Matear M, Lambert G, Allen AM, & May CN (2019). Neurohumoral interactions contributing to renal vasoconstriction and decreased renal blood flow in heart failure. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 317, R386–R396. [DOI] [PubMed] [Google Scholar]
- Rotto DM, & Kaufman MP (1988). Effects of metabolic products of muscular contraction on the discharge of group III and IV afferents. Journal of Applied Physiology, 64, 2306–2313. [DOI] [PubMed] [Google Scholar]
- Ruan N, Tribble J, Peterson AM, Jiang Q, Wang JQ, & Chu XP (2021). Acid-sensing ion channels and mechanosensation. International Journal of Molecular Sciences 4810, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ES, Cadiou H, & McNaughton PA (2007). Arachidonic acid potentiates acid-sensing ion channels in rat sensory neurons by a direct action. Neuroscience, 145, 686–698. [DOI] [PubMed] [Google Scholar]
- Smith JR, Joyner MJ, Curry TB, Borlaug BA, Keller-Ross ML, Van Iterson EH, & Olson TP (2020). Locomotor muscle group III/IV afferents constrain stroke volume and contribute to exercise intolerance in human heart failure. Journal of Physiology, 598, 5379–5390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SA, Mammen PP, Mitchell JH, & Garry MG (2003). Role of the exercise pressor reflex in rats with dilated cardiomyopathy. Circulation, 108, 1126–1132. [DOI] [PubMed] [Google Scholar]
- Smith SA, Mitchell JH, & Garry MG (2001). Electrically induced static exercise elicits a pressor response in the decerebrate rat. Journal of Physiology, 537, 961–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SA, Mitchell JH, Naseem RH, & Garry MG (2005a). Mechanoreflex mediates the exaggerated exercise pressor reflex in heart failure. Circulation, 112, 2293–2300. [DOI] [PubMed] [Google Scholar]
- Smith SA, Williams MA, Mitchell JH, Mammen PP, & Garry MG (2005b). The capsaicin-sensitive afferent neuron in skeletal muscle is abnormal in heart failure. Circulation, 111, 2056–2065. [DOI] [PubMed] [Google Scholar]
- Stebbins CL, Brown B, Levin D, & Longhurst JC (1988). Reflex effect of skeletal muscle mechanoreceptor stimulation on the cardiovascular system. Journal of Applied Physiology, 65, 1539–1547. [DOI] [PubMed] [Google Scholar]
- Sterns DA, Ettinger SM, Gray KS, Whisler SK, Mosher TJ, Smith MB, & Sinoway LI (1991). Skeletal muscle metaboreceptor exercise responses are attenuated in heart failure. Circulation, 84, 2034–2039. [DOI] [PubMed] [Google Scholar]
- Stickland MK, Miller JD, Smith CA, & Dempsey JA (2007). Carotid chemoreceptor modulation of regional blood flow distribution during exercise in health and chronic heart failure. Circulation Research, 100, 1371–1378. [DOI] [PubMed] [Google Scholar]
- Stone AJ, Copp SW, Kim JS, & Kaufman MP (2015). Combined, but not individual, blockade of ASIC3, P2X, and EP4 receptors attenuates the exercise pressor reflex in rats with freely perfused hindlimb muscles. Journal of Applied Physiology, 119, 1330–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange S, Secher NH, Pawelczyk JA, Karpakka J, Christensen NJ, Mitchell JH, & Saltin B (1993). Neural control of cardiovascular responses and of ventilation during dynamic exercise in man. Journal of Physiology, 470, 693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchimochi H, McCord JL, & Kaufman MP (2010). Peripheral mu-opioid receptors attenuate the augmented exercise pressor reflex in rats with chronic femoral artery occlusion. American Journal of Physiology. Heart and Circulatory Physiology, 299, H557–H565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchimochi H, Yamauchi K, McCord JL, & Kaufman MP (2011). Blockade of acid sensing ion channels attenuates the augmented exercise pressor reflex in rats with chronic femoral artery occlusion. Journal of Physiology, 589, 6173–6189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor RG, Bertocci LA, Pryor SL, & Nunnally RL (1988). Sympathetic nerve discharge is coupled to muscle cell pH during exercise in humans. Journal of Clinical Investigation, 82, 1301–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voilley N, de Weille J, Mamet J, & Lazdunski M (2001). Nonsteroid anti-inflammatory drugs inhibit both the activity and the inflammation-induced expression of acid-sensing ion channels in nociceptors. Journal of Neuroscience, 21, 8026–8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walder RY, Rasmussen LA, Rainier JD, Light AR, Wemmie JA, & Sluka KA (2010). ASIC1 and ASIC3 play different roles in the development of Hyperalgesia after inflammatory muscle injury. Journal of Pain, 11, 210–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HJ, Li YL, Gao L, Zucker IH, & Wang W (2010a). Alteration in skeletal muscle afferents in rats with chronic heart failure. Journal of Physiology, 588, 5033–5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HJ, Pan YX, Wang WZ, Gao L, Zimmerman MC, Zucker IH, & Wang W (2010b). Exercise training prevents the exaggerated exercise pressor reflex in rats with chronic heart failure. Journal of Applied Physiology, 108, 1365–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing J, Lu J, & Li J (2015). ASIC3 contributes to the blunted muscle metaboreflex in heart failure. Medicine and Science in Sports and Exercise, 47, 257–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi J, Wenk HN, Naves LA, & McCleskey EW (2006). Sustained currents through ASIC3 ion channels at the modest pH changes that occur during myocardial ischemia. Circulation Research, 99, 501–509. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


