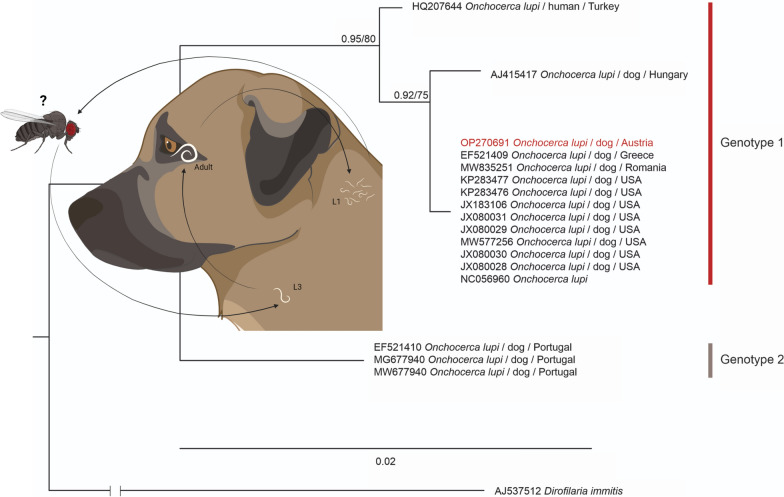
Abstract
Onchocerca lupi is an emerging canine ocular pathogen with zoonotic potential. In Europe, known endemic areas are the Iberian Peninsula and Greece, but the parasite has also been found in Romania, Hungary, and Germany. A 5-year-old Irish Wolfhound was presented in August 2021 with ocular discharge. A subconjunctival granulomatous nodule containing several nematode fragments was removed. Molecular analysis of the mitochondrial cytochrome c oxidase subunit I gene confirmed the presence of O. lupi genotype 1. This is the first report of autochthonous O. lupi infection in a dog from Austria.
Graphical Abstract
Keywords: Canine onchocercosis, Cytochrome c oxidase subunit I gene (COI), Ocular helminthosis, PCR, Zoonotic
Background
Species of the family Onchocercidae parasitize many different vertebrate hosts and include pathogens relevant to human health such as Onchocerca volvulus, the causative agent of river blindness [1]. Onchocerca lupi was first described in a wolf (Canis lupus) from Russia in 1967 and affects dogs (Canis lupus familiaris) and, to a lesser degree, cats (Felis silvestris catus). Moreover, humans can be infected as well [2–5]. The adult worm is most frequently found in the subconjunctival or subcutaneous tissue, but in humans spinal cord infections have been also reported [5–7]. Clinical signs may vary, and animals that present no obvious clinical signs may not be diagnosed for several years [8]. Based on the vector capacity of other Onchocerca spp. and the findings of O. lupi DNA in Simuliidae, these have been suggested as potential vectors [9]. Other arthropods have also been considered, but evidence of competent transmission is still missing [10–12]. This parasite has been documented in Europe, America, Africa, and Asia [1, 13–15]. In Europe, the Iberian Peninsula and Greece are known to be endemic areas, but cases have also been reported from Romania, Hungary, and Germany [8, 16–19]. Diagnosis can be based on adult specimen identification in clinical cases or by skin snips and detection of microfilariae [17, 20, 21]. Morphological identification can be confirmed by PCR of, for example, the mitochondrial cytochrome c oxidase subunit I (COI) gene [22]. Treatment recommendations include surgical removal of the parasite and the use of drugs such as macrocyclic lactones [1, 23]. The present report describes the first autochthonous O. lupi infection in Austria.
Methods
In August 2021 a 5-year-old Irish Wolfhound living in Güssing district (Burgenland), which was born in Austria and had never left the country, was presented with ocular discharge. No other clinical signs were noted at physical examination. Subconjunctival granulomatous nodules containing nematodes were detected in both eyes and removed with forceps. The nodules were placed in saline solution and sent to the University of Veterinary Medicine, Vienna, where it was stored at −20 °C until further analysis. Nematodes were examined morphologically under a stereomicroscope, and DNA was extracted from fragments using a commercial DNA extraction kit (DNeasy® Blood & Tissue Kit; QIAGEN, Hilden, Germany) according to the manufacturer's instructions. To obtain a fragment of the COI gene with 649 nucleotide positions, PCR was done on a fragment of one nematode using primers COIintF/COIintR [24] with the following amplifying temperature profile: initial denaturation at 95 °C for 2 min, followed by 35 cycles of 95 °C, 50 °C, and 72 °C each for 1 min, and final extension at 72 °C for 7 min. PCR products were run on 2% agarose gels stained with Midori Green. The PCR product was further analysed by Sanger sequencing (LGC Genomics, Berlin, Germany). The sequence was compared to available sequences using the BOLD and GenBank nucleotide basic local alignment search tool.
For phylogenetic analysis, nucleotide sequences of O. lupi available on the NCBI GenBank database were searched by using the BLAST function, using the sequence obtained in this study. The sequences were aligned and sorted using the default option (FFT–NS–2) in MAFFT v.7.311 [25], and sequences not covering the fragment of the sequences obtained in this study were excluded. Maximum likelihood (ML) and Bayesian inference (BI) trees were calculated based on the alignment, including 17 sequences (649 nucleotide positions). Sequences were collapsed to haplotypes using DAMBE v.7.0.5.1 [26], leaving four haplotypes. As outgroup, a sequence of Dirofilaria immitis (GenBank accession number: AJ537512) was used. ML bootstrap consensus trees (1000 replicates) were calculated using the W-IQ-TREE web server (http://iqtree.cibiv.univie.ac.at/; [27]) applying the model TIM3 + F + G4, which were suggested as best fit for the data set in the model test according to the corrected Akaike information criterion. The BI trees were calculated using MrBayes v.3.2.7 [28], applying the next complex model GTR + G, because the same model was not available in this program. The analysis was run for 106 generations (number of chains: 4), sampling every 1000th tree. The first 25% of trees were discarded as burn-in, and a 50% majority-rule consensus tree was calculated based on the remaining 7500 trees.
Results and discussion
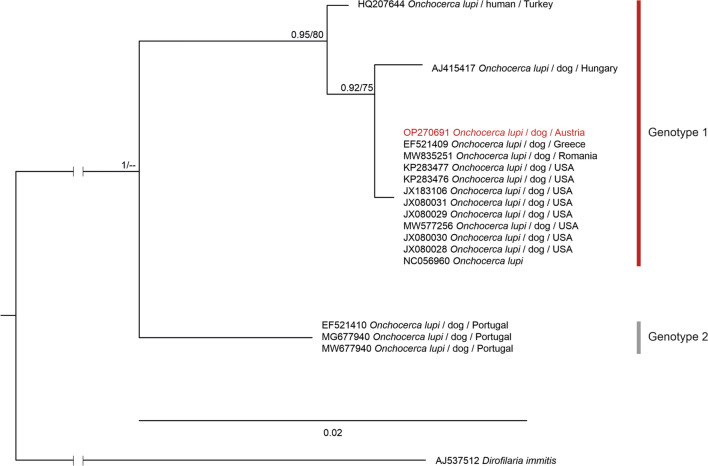
The dog was treated twice at an interval of 2 weeks with a combination compound containing moxidectin (2.5 mg/kg BW) and imidacloprid (10 mg/kg BW) Spot-On (Advocate®; Bayer AG, Leverkusen, Germany) for the control of O. lupi and with a topical ointment containing tobramycin and dexamethasone (Tobradex®, Novartis AG, Basel, Switzerland) to promote the healing of the eye inflammation. The clinical signs disappeared and did not recur within the follow-up period of 1 year. Treatment with moxidectin has been reported to be successful [23]. However, whether medical treatment or the surgical removal alone resolved clinical signs cannot be concluded with certainty. In addition, it is not clear whether the treatment eliminated all nematodes as no skin biopsies could be obtained before and after treatment because of the lack of owner consent. Morphological examination of the nodule revealed several worm fragments. The DNA sequence obtained was 100% identical to an O. lupi sequence documented in a dog from Greece (GenBank accession number: EF521409) and has been uploaded to BoldSystems® (Process ID: PAVEA164-22) and GenBank (accession number: OP270691). This haplotype has been referred to as genotype 1 (Fig. 1), which occurs in northern America, southwestern Asia, and Europe, with the exception of the Iberian Peninsula, where genotype 2 is present [15].
Fig. 1.
Bayesian interference (BI) tree featuring mitochondrial cytochrome c oxidase subunit I (COI) sequences (649 nucleotide positions) of Onchocerca lupi. Nodes are marked with BI posterior probabilities and ML bootstrap values. The sequence marked in red was obtained in the present study. Scale bar indicates the expected mean number of substitutions per site according to the model of sequence evolution applied
In total, 45 species of Simuliidae, which could potentially act as vectors, are known to exist in Austria [29, 30]. In the Lafnitz River near Heiligenkreuz town (Burgenland), located near Güssing (Burgenland), Simulium erythrocephalum, Simulium ibariense, and Simulium ornatum have been found [29].
Coyotes (Canis latrans) have been considered as reservoir hosts in America [31]. In Europe, coyotes are not present, but other wild canids could probably fulfil this role. Another more likely mode of introduction is through pets travelling from endemic regions and subsequent establishment of the parasite in areas where it has not been present before [32]. To determine the current prevalence of O. lupi in Austria, a prevalence study should be performed in dogs and/or wild canids using skin snips and/or serology [8, 17, 33].
Conclusion
Information on the treatment but also on transmission and distribution of this parasite is still scarce. This case report highlights that O. lupi can also be present in countries not yet classified as endemic and underlines the need to raise awareness of this zoonotic parasite.
Acknowledgements
The authors thank Josef Harl for his supervision in generating the phylogenetic tree.
Abbreviations
- BI
Bayesian inference
- COI
Mitochondrial cytochrome c oxidase subunit I
- ML
Maximum likelihood
Author contributions
MSU: concept and design, analysis of samples and data, drafting the manuscript. AH: concept and design, acquisition of samples, medical care and report, revising the manuscript. KS: concept and design, revising the manuscript. HPF: concept and design, supervision, analysis of data, revising the manuscript. All authors have read and approved the final manuscript.
Funding
Open Access funding for this article was provided by the University of Veterinary Medicine Vienna (Vetmeduni Vienna). Financial support for was provided by Boehringer Ingelheim.
Availability of data and materials
Additional data can be provided on request.
Declarations
Ethics approval and consent to participate
The authors confirm that the ethical policies of the journal, as noted on the journal’s author guidelines page, have been adhered to. No ethical approval was required as the obtained sample was gathered through a procedure necessary for medical reasons.
Consent for publication
Not applicable.
Competing interests
None declared.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Maria Sophia Unterköfler, Email: maria.unterkoefler@vetmeduni.ac.at.
Alexandra Huck, Email: ordination@drhuck.at.
Katja Silbermayr, Email: katja.silbermayr@boehringer-ingelheim.com.
Hans-Peter Fuehrer, Email: Hans-Peter.Fuehrer@vetmeduni.ac.at.
References
- 1.Grácio AJS, Richter J, Komnenou AT, Grácio MA. Onchocerciasis caused by Onchocerca lupi: an emerging zoonotic infection. Systematic review Parasitol Res. 2015;114:2401–2413. doi: 10.1007/s00436-015-4535-7. [DOI] [PubMed] [Google Scholar]
- 2.Bergua A, Hohberger B, Held J, Muntau B, Tannich E, Tappe D. Human case of Onchocerca lupi infection, Germany, August 2014. Euro Surveill. 2015;20:21099. doi: 10.2807/1560-7917.es2015.20.16.21099. [DOI] [PubMed] [Google Scholar]
- 3.Otranto D, Sakru N, Testini G, Gürlü VP, Yakar K, Lia RP, et al. Case report: First evidence of human zoonotic infection by Onchocerca lupi (Spirurida, Onchocercidae) Am J Trop Med Hyg. 2011;84:55–58. doi: 10.4269/ajtmh.2011.10-0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodojana TE. A new species of nematode, Onchocerca lupi n. sp., from Canis lupus cubanensis. Soobshchenyia Akad Nauk. 1967;45:715–9. [Google Scholar]
- 5.Rojas A, Morales-Calvo F, Salant H, Otranto D, Baneth G. Zoonotic ocular onchocercosis by Onchocerca lupi. Yale J Biol Med. 2021;94:331–341. [PMC free article] [PubMed] [Google Scholar]
- 6.Chen T, Moon K, deMello DE, Feiz-Erfan I, Theodore N, Bhardwaj RD. Case report of an epidural cervical Onchocerca lupi infection in a 13-year-old boy. J Neurosurg Pediatr. 2015;16:217–221. doi: 10.3171/2014.12.PEDS14462. [DOI] [PubMed] [Google Scholar]
- 7.Dudley RWR, Smith C, Dishop M, Mirsky D, Handler MH, Rao S. A cervical spine mass caused by Onchocerca lupi. Lancet. 2015;386:1372. doi: 10.1016/S0140-6736(14)62255-8. [DOI] [PubMed] [Google Scholar]
- 8.Otranto D, Dantas-Torres F, Giannelli A, Latrofa MS, Papadopoulos E, Cardoso L, et al. Zoonotic Onchocerca lupi infection in dogs, Greece and Portugal, 2011–2012. Emerg Infect Dis. 2013;19:2000–2003. doi: 10.3201/eid1912.130264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hassan HK, Bolcen S, Kubofcik J, Nutman TB, Eberhard ML, Middleton K, et al. Isolation of Onchocerca lupi in dogs and black flies, California, USA. Emerg Infect Dis. 2015;21:789–796. doi: 10.3201/eid2105.142011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brilhante AF, de Albuquerque AL, de Rocha ACB, Ayres CFJ, Paiva MHS, de Ávila MM, et al. First report of an Onchocercidae worm infecting Psychodopygus carrerai carrerai sandfly, a putative vector of Leishmania braziliensis in the Amazon. Sci Rep. 2020;10:15246. doi: 10.1038/s41598-020-72065-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manoj RRS, Latrofa MS, Cavalera MA, Mendoza-Roldan JA, Maia C, Otranto D. Molecular detection of zoonotic filarioids in Culex spp. from Portugal. Med Vet Entomol. 2021;35:468–77. doi: 10.1111/mve.12524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Otranto D, Dantas-Torres F, Papadopoulos E, Petrić D, Ćupina AI, Bain O. Tracking the vector of Onchocerca lupi in a rural area of Greece. Emerg Infect Dis. 2012;18:1196–1200. doi: 10.3201/eid1807.AD1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Labelle AL, Maddox CW, Daniels JB, Lanka S, Eggett TE, Dubielzig RR, et al. Canine ocular onchocercosis in the United States is associated with Onchocerca lupi. Vet Parasitol. 2013;193:297–301. doi: 10.1016/j.vetpar.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 14.Otranto D, Dantas-Torres F, Cebeci Z, Yeniad B, Buyukbabani N, Boral OB, et al. Human ocular filariasis: further evidence on the zoonotic role of Onchocerca lupi. Parasit Vectors. 2012;5:84. doi: 10.1186/1756-3305-5-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rojas A, Salant H, Yasur-Landau D, Tsarfati H, Baneth G. First report of Onchocerca lupi from Israel and confirmation of two genotypes circulating among canine, feline and human hosts. Parasitology. 2020;147:1723–1727. doi: 10.1017/S0031182020001560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hermosilla C, Hetzel U, Bausch M, Grübl J, Bauer C. First autochthonous case of canine ocular onchocercosis in Germany. Vet Rec. 2005;156:450–452. doi: 10.1136/vr.156.14.450. [DOI] [PubMed] [Google Scholar]
- 17.Miró G, Montoya A, Checa R, Gálvez R, Mínguez JJ, Marino V, et al. First detection of Onchocerca lupi infection in dogs in southern Spain. Parasit Vectors. 2016;9:290. doi: 10.1186/s13071-016-1587-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Széll Z, Sréter T, Erdélyi I, Varga I. Ocular onchocercosis in dogs: aberrant infection in an accidental host or lupi onchocercosis? Vet Parasitol. 2001;101:115–125. doi: 10.1016/S0304-4017(01)00507-6. [DOI] [PubMed] [Google Scholar]
- 19.Tudor P, Turcitu M, Mateescu C, Dantas-Torres F, Tudor N, Bărbuceanu F, et al. Zoonotic ocular onchocercosis caused by Onchocerca lupi in dogs in Romania. Parasitol Res. 2016;115:859–862. doi: 10.1007/s00436-015-4816-1. [DOI] [PubMed] [Google Scholar]
- 20.Egyed Z, Sréter T, Széll Z, Beszteri B, Oravecz O, Márialigeti K, et al. Morphologic and genetic characterization of Onchocerca lupi infecting dogs. Vet Parasitol. 2001;102:309–319. doi: 10.1016/S0304-4017(01)00541-6. [DOI] [PubMed] [Google Scholar]
- 21.Otranto D, Dantas-Torres F, Giannelli A, Abramo F, Ignjatović Ćupina A, Petrić D, et al. Cutaneous distribution and circadian rhythm of Onchocerca lupi microfilariae in dogs. PLoS Negl Trop Dis. 2013;7:e2585. doi: 10.1371/journal.pntd.0002585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Egyed Z, Sréter T, Széll Z, Nyirő G, Márialigeti K, Varga I. Molecular phylogenetic analysis of Onchocerca lupi and its Wolbachia endosymbiont. Vet Parasitol. 2002;108:153–161. doi: 10.1016/s0304-4017(02)00186-3. [DOI] [PubMed] [Google Scholar]
- 23.Otranto D, Colella V, Bezerra-Santos MA, Mendoza-Roldan JA, Cavalera MA, Pereira A, et al. Efficacy of a spot-on formulation containing moxidectin 2.5%/imidacloprid 10% for the treatment of Cercopithifilaria spp. and Onchocerca lupi microfilariae in naturally infected dogs from Portugal. Parasit Vectors. 2021;14:199. doi: 10.1186/s13071-021-04704-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Casiraghi M, Anderson TJ, Bandi C, Bazzocchi C, Genchi C. A phylogenetic analysis of filarial nematodes: comparison with the phylogeny of Wolbachia endosymbionts. Parasitology. 2001;122:93–103. doi: 10.1017/S0031182000007149. [DOI] [PubMed] [Google Scholar]
- 25.Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xia X, Xie Z. DAMBE: Software package for data analysis in molecular biology and evolution. J Hered. 2001;92:371–373. doi: 10.1093/jhered/92.4.371. [DOI] [PubMed] [Google Scholar]
- 27.Trifinopoulos J, Nguyen L-T, von Haeseler A, Minh BQ. W-IQ-TREE: a fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 2016;44:W232–W235. doi: 10.1093/nar/gkw256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, et al. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol. 2012;61:539–42. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Car M, Lechthaler W. First records of Simulium (Hellichiella) latipes (Meigen), Simulium ibariense Zivkovich & Grenier, Simulium codreanui (Sherban) and the occurrence of Simulium bezzii (Corti) (Diptera: Simuliidae) in Austria. Limnologica. 2002;32:248–254. doi: 10.1016/S0075-9511(02)80031-7. [DOI] [Google Scholar]
- 30.Ebmer D, Balfanz F, Voracek T, Hering-Hagenbeck S, Pichler-Scheder C, Walochnik J, et al. The Palearctic blackfly Simulium equinum (Diptera: Simuliidae) as a biting pest of captive nyala antelopes (Tragelaphus angasii) Zoo Biol. 2022 doi: 10.1002/zoo.21719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roe CC, Yaglom H, Howard A, Urbanz J, Verocai GG, Andrews L, et al. Coyotes as reservoirs for Onchocerca lupi , United States, 2015–2018. Emerg Infect Dis. 2020;26:2989–2993. doi: 10.3201/eid2612.190136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Colella V, Lia RP, Di Paola G, Cortes H, Cardoso L, Otranto D. International dog travelling and risk for zoonotic Onchocerca lupi. Transbound Emerg Dis. 2018;65:1107–1109. doi: 10.1111/tbed.12842. [DOI] [PubMed] [Google Scholar]
- 33.Giannelli A, Cantacessi C, Graves P, Becker L, Campbell BE, Dantas-Torres F, et al. A preliminary investigation of serological tools for the detection of Onchocerca lupi infection in dogs. Parasitol Res. 2014;113:1989–1991. doi: 10.1007/s00436-014-3844-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Additional data can be provided on request.