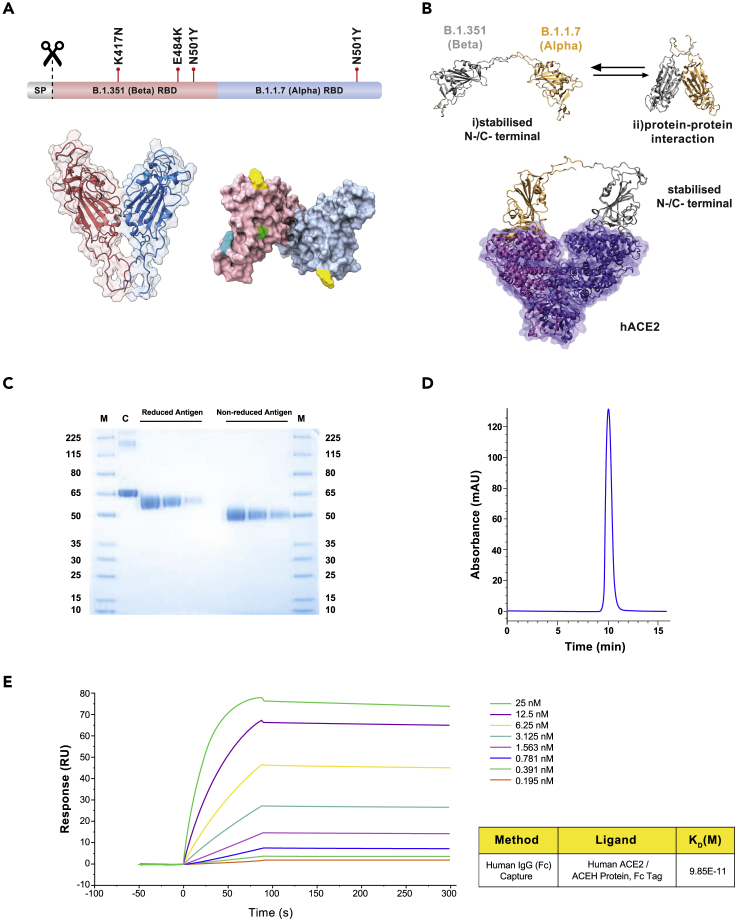
Figure 1.
Structure and characterization of the B.1.351 (Beta) - B.1.1.7 (Alpha) receptor-binding domain (RBD) heterodimer, immunogen of PHH-1V
(A) Structural representation of the RBD heterodimer. Top: sequence diagram. Bottom left: front view of the RBD heterodimer cartoon structure. Bottom right: top view of the antigen surface structure. Mutations are highlighted in green (K417N), cyan (E484K) and yellow (N501Y).
(B) Computation modeling for PHH-1V vaccine. Top: AlphaFold2 results for the B.1.351-B.1.1.7 construct. This highlights the presence of two different construct conformations: (1) Stabilized N-/C-terminal conformation and (2) adopting protein-protein interactions. Bottom: hACE2 receptor-construct model derived from MD calculations of the B.1.351-B.1.1.7 construct. RBD residues 1 to 219 and 220 to 439 are shown in gray and orange, respectively, whereas ACE2 monomers are shown as a transparent surface and cartoon representation in violet and purple.
(C) SDS-PAGE. The reduced and non-reduced purified antigens were loaded at three serial dilutions: 1/10, 1/20 and 1/40. M: molecular weight ladder. C: BSA control.
(D) SEC-HPLC chromatogram of the purified antigen.
(E) Surface plasmon resonance (SPR) for the quantitative evaluation of the affinity between the antigen and its natural ligand, the human ACE2 receptor. RU: resonance units.