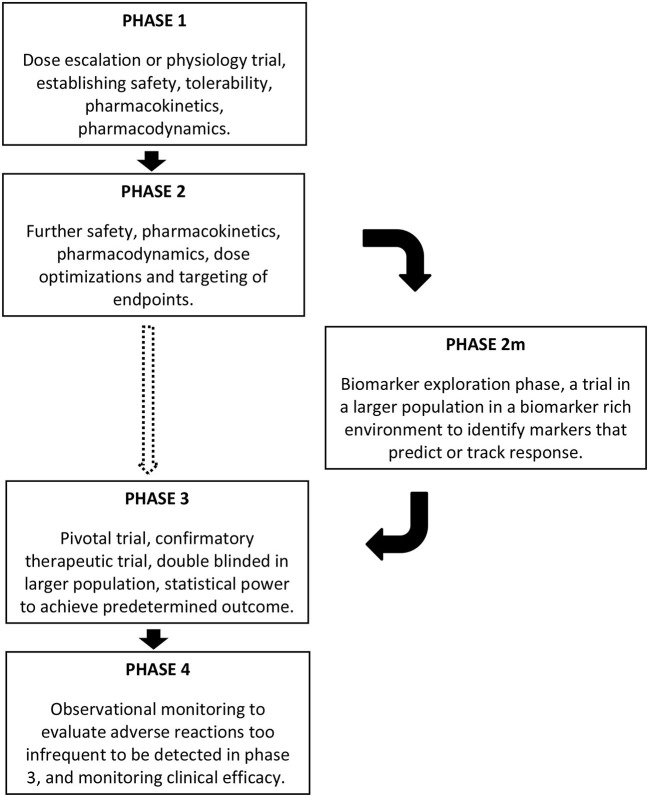
Figure 1.
Schematic representing the location of a phase 2m in clinical trials, after phase 1 and phase 2, and before phase 3, if the need for a biomarker exploration phase is warranted based on the nature of the clinical condition. The dashed arrow moving directly from phase 2 to phase 3 might be appropriate for some conditions, but incorporating the phase 2m seems warranted in conditions such as ASD.