Fig. 1.
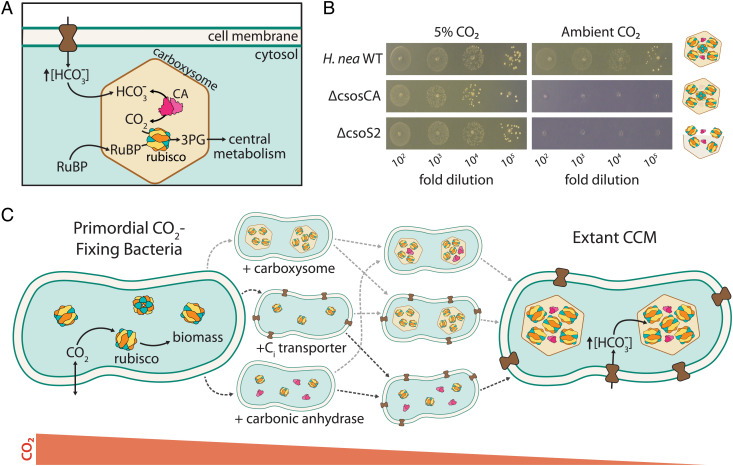
Mechanism and potential routes for the evolution of the bacterial CO2-concentrating mechanism. (A) Today, the bacterial CCM functions through the concerted action of three primary features - (i) an inorganic carbon (Ci) transporter at the cell membrane, and (ii) a properly-formed carboxysome structure (iii) co-encapsulating rubisco with carbonic anhydrase (CA). Ci uptake leads to a high intracellular HCO3− concentration, well above equilibrium with the external environment. Elevated HCO3− is converted to a high carboxysomal CO2 concentration by CA activity located only there, which promotes carboxylation by rubisco. (B) Mutants lacking genes coding for essential CCM components grow in elevated CO2 but fail to grow in ambient air, as shown here for mutations to the α-carboxysome in the proteobacterial chemoautotroph H. neapolitanus. Strains lacking the carboxysomal CA (ΔcsosCA) or an unstructured protein required for carboxysome formation (Δcsos2) failed to grow in ambient air, but grew robustly in 5% CO2 (>108 colony-forming units/ml, SI Appendix, Fig. S1). See SI Appendix, Table S4 for description of mutant strains. (C) We consider the CCM to be composed of three functionalities beyond rubisco itself: a CA enzyme (magenta), a Ci transporter (dark brown), and carboxysome encapsulation of rubisco with CA (light brown). If CO2 levels were sufficiently high, primordial CO2-fixing bacteria would not have needed a CCM. We sought to discriminate experimentally between the six sequential trajectories (dashed arrows) in which CCM components could have been acquired.