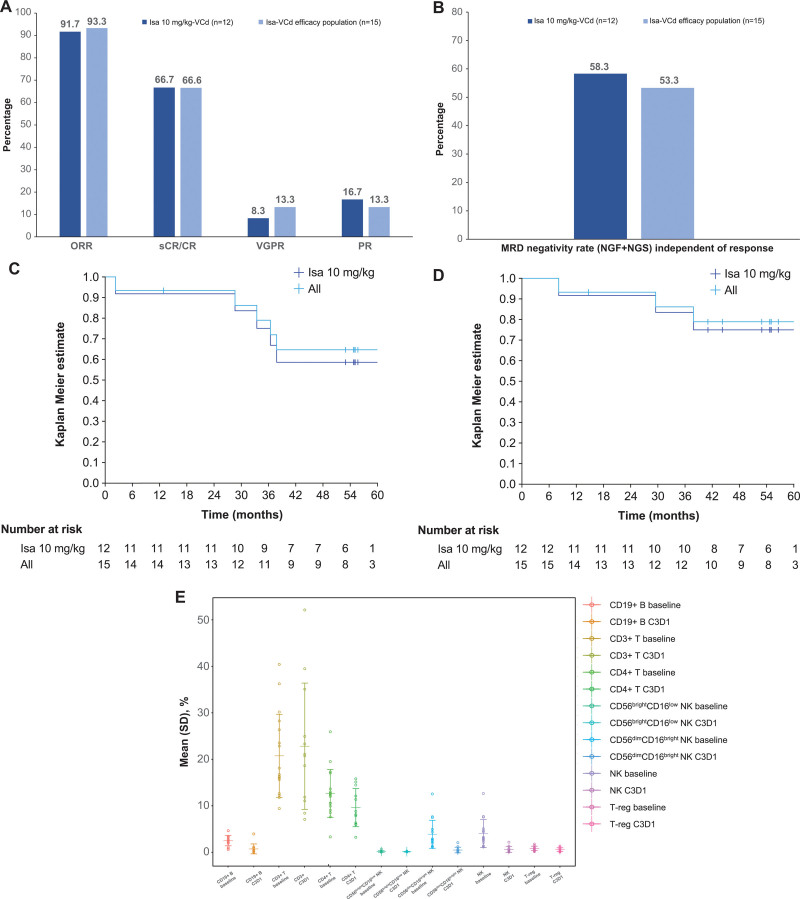
Figure 1.
Best overall response (A), PFS (B), OS (C), MRD (D), and blood cell immunophenotyping results at baseline and day 1/cycle 3 (predose) (E). (A–D) Patients included in efficacy population (n = 15): Isa 10 mg/kg-VCd, n = 12; Isa 20 mg/kg-VCd, n = 3. All = all patients in the efficacy population. MRD was evaluated in patients with ≥VGPR by NGF and NGS methods at 10−5 and MRD negativity rate determined by combining both methods in the case of at least 1 method yielding negative results and the other method showing no positive result at the same time. (E) Patients included in baseline population: Isa 10 mg/kg–VCd, n = 13; Isa 20 mg/kg–VCd, n = 4 and in on-treatment population: Isa 10 mg/kg-VCd, n = 10; Isa 20 mg/kg-VCd, n = 2. Results expressed as percentage of leukocytes. C = cyclophosphamide; C3D1 = cycle 3 day 1; CR = complete response; d = dexamethasone; Isa = isatuximab; MRD = minimal residual disease; NGF = next-generation flow; NGS = next-generation sequencing; NK = natural killer; ORR = overall response rate; OS = overall survival; PFS = progression-free survival; PR = partial response; sCR = stringent CR; SD = standard deviation; T-reg = regulatory T cell; V = bortezomib; VGPR = very good partial response.