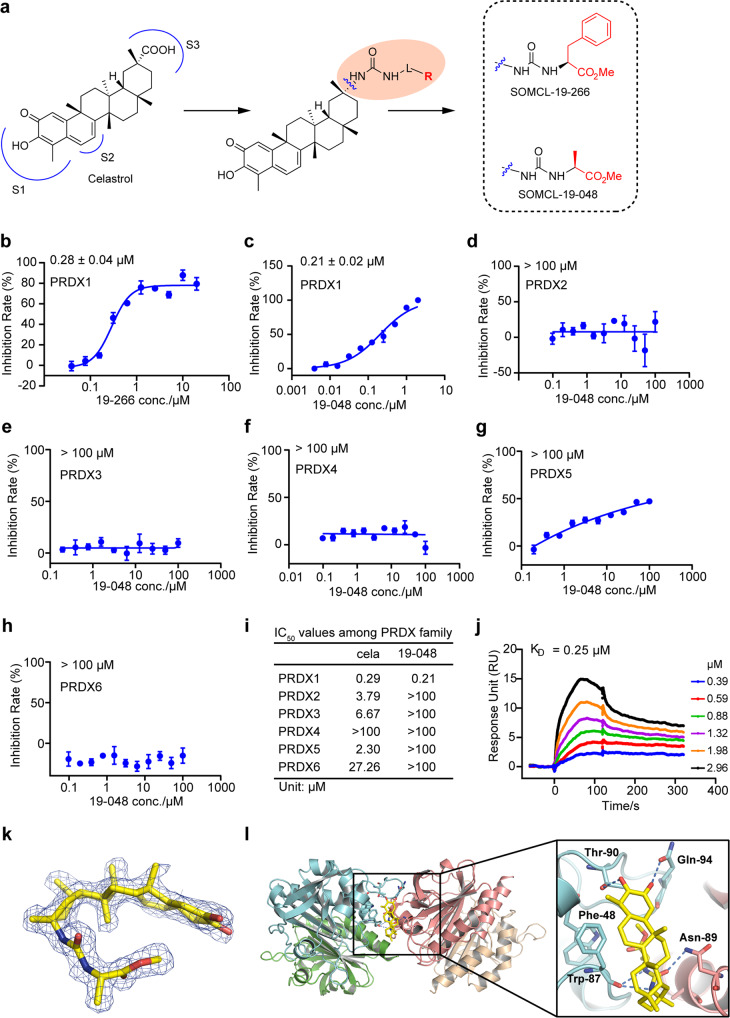
Fig. 4.
New derivative compound of Celastrol named 19-048 showed improved potency and selectivity. a The scheme of synthesis for new derivative compounds of Celastrol. Carboxyl group of Celastrol was substituted by guanidine group. Compound 19-266 and 19-048 were synthesized by guanidine terminal substitution. b Peroxidase activity inhibition of PRDX1 by compound 19-266. Recombinant PRDX1 was incubated with different concentrations of compound for 1.5 h. The inhibition rate was calculated from initial reaction slope of each assay well as shown in Supplementary Fig. 4a. The IC50 value of compound 19-266 against PRDX1 is 0.28 ± 0.040 µM. All data is shown in mean ± SEM. Peroxidase activity inhibition of PRDX1 (c), PRDX2 (d), PRDX3 (e), PRDX4 (f), PRDX5 (g), PRDX6 (h) incubated with compound 19-048. The inhibition rates were calculated from initial reaction slope of figure S4b-S4g for PRDX1~PRDX6. The IC50 value of compound 19-048 against PRDX1 is 0.21 ± 0.02 µM, while the IC50 values of compound 19-048 against PRDX2~6 are above 100 µM. The inhibitions of peroxidase activity assay were calculated from triplicate experiments. All data is shown in mean ± SEM. i Summary of IC50 values for Celastrol and its derivative compound 19-048 against the six proteins from human PRDX family. j The binding affinity of compound 19-048 with recombinant PRDX1 determined by the SPR assay. k The FO–FC electron density map of compound 19-048 contoured at 2.5 σ. l Binding mode of compound 19-048 with PRDX1C52SC83S,1-175aa homodimers. Compound 19-048 is shown as yellow sticks