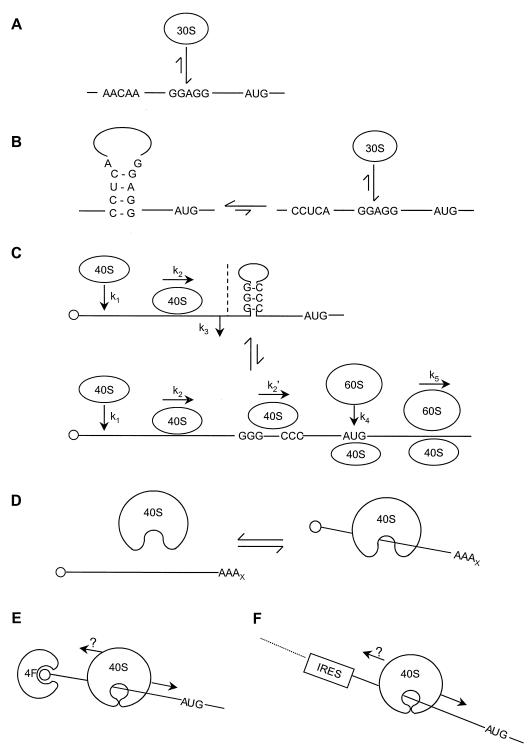
FIG. 10.
Principles of kinetic control affecting prokaryotic and eukaryotic initiation. (A) A prokaryotic 30S ribosomal subunit has direct access to the SD sequence of an mRNA with an unstructured TIR. (B) Inhibition via structure in the TIR can be adequately modelled by assuming a thermodynamic control mechanism in which the steady-state distribution of folded and unfolded TIR dictates the amount of mRNA accessible to ribosome binding. (C) In the eukaryotic case, 40S ribosomal subunit binding can occur unhindered on a leader bearing localized structure, but the structured region is thought then to inhibit the scanning process. The off-rate (k3) for ribosomal subunits in this situation is unknown. Disruption of the secondary structure can be driven by an apparently ATP-dependent process, allowing resumption of scanning through the structured region. (D) Random internal binding of 40S ribosomal subunits to mRNA seems possible but is most probably not favored kinetically, and there may be kinetic or mechanistic restrictions on the ability of the subunits to become tightly associated with the mRNA. Initiation factors will influence the type of interaction entered into (compare Fig. 4). (E and F) 5′-end-dependent (E) or IRES-directed (F) initiation results in a tight association between the 40S subunit and the mRNA (the clasp is closed around the mRNA). It is not known whether scanning is normally unidirectional (E and F).