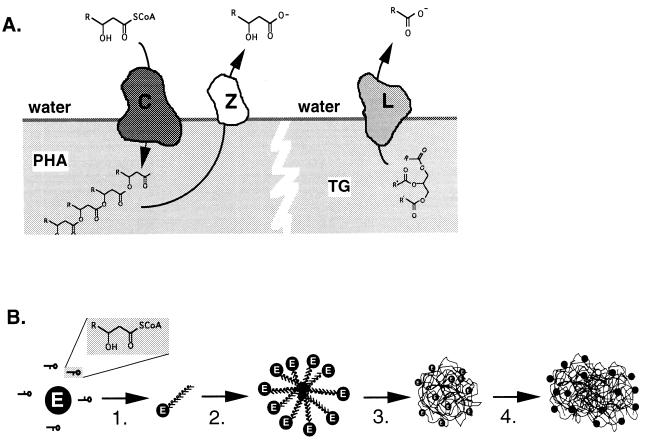
FIG. 6.
(A) Similarities between PHA polymerase and lipase. PHA polymerase (C) acts at the surface of a PHA granule, where soluble precursors are polymerized and deposited in the hydrophobic environment of the granule. PHA depolymerase (Z) also acts at this surface and liberates the monomers from the polymer. Both enzymatic reactions are reminiscent of that of lipase (L), which cleaves ester bonds at triglyceride (TG)/water interfaces, yielding free acids and alkanols. (B) Proposed mechanism for the formation of PHA granules. Soluble enzyme converts monomer-CoA to oligomers, which remain enzyme bound (step 1). At a critical oligomer length and enzyme-oligomer concentration, the enzyme-oligomer complexes form micelles with the enzyme located at the interface, separating the PHA from the cytosol (step 2). Because of this compartmentalization, PHA polymerization is facilitated. Because the hydrophobic polymer can now be extruded into a hydrophobic environment instead of the aqueous phase, the reaction proceeds faster. The micelles are expanded and now appear as intracellular, granular structures visible with the phase-contrast microscope (step 3). As the number of granules increase, they may fuse and coalesce, giving rise to large aggregates of PHA (step 4).