Figure 1. Sources and mechanisms of inflammation and its effects on neurotransmitters and circuits that contribute to psychiatric symptoms.
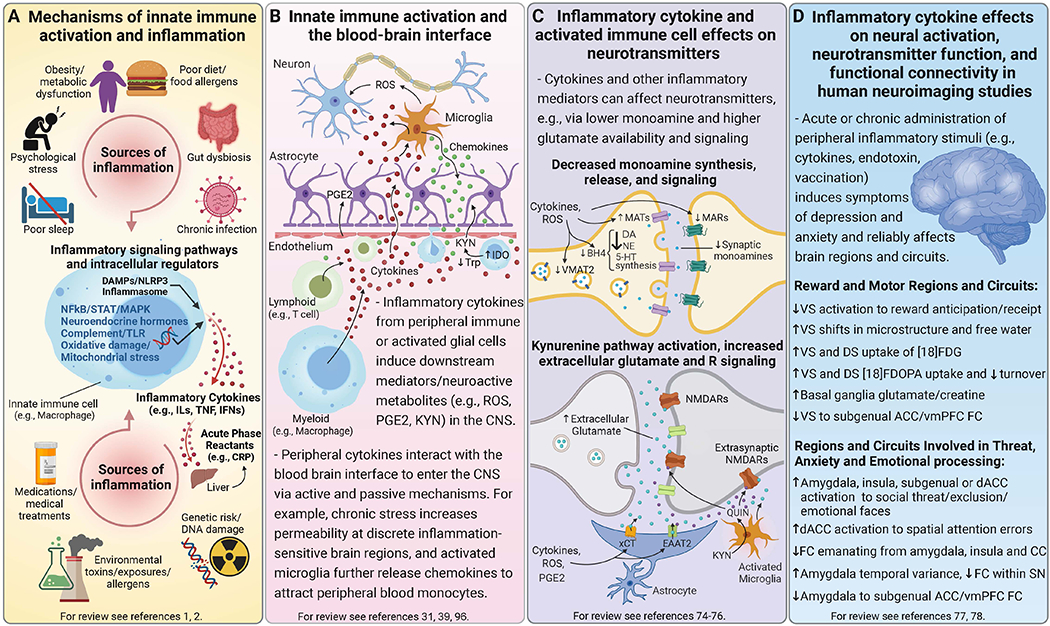
Mechanisms of innate immune activation and chronic low-grade inflammation. Panel A. Genetic predisposition may interact with multiple environmental and lifestyle factors that contribute to chronic low-grade inflammation, many of which are risk factors for both psychiatric disorders and major medical illnesses including psychological stress, disturbed sleep, poor diet, metabolic changes and gut dysbiosis, as well as chronic infections and environmental toxins. Innate immune cells are activated by pathogens or sterile inflammatory (e.g., DAMPs, metabolic, neuroendocrine, or oxidative stress) pathways to synthesize and release inflammatory mediators like cytokines (e.g., ILs, TNF, IFNs), which in turn induce acute phase reactants such as CRP from the liver. Acute inflammatory activity is typically resolved by homeostatic processes, but disruption of these mechanisms or prolonged immune activation can lead to chronic low-grade inflammation that impacts multiple systems including the brain. Bidirectional inflammatory processes at the blood-brain interface. Panel B. Activated innate immune cells interact with adaptive immune cells (e.g., lymphocytes), migrate into circulation, and traffic to organs and tissues including the brain. Circulating inflammatory cytokines and activated immune cells communicate with brain endothelial cells to induce other inflammatory mediators (e.g., PGE2). Inflammatory cytokines can enter the CNS via active transport or passively at circumventricular organs or openings in tight junctions of the BBB, while also signaling to the brain via vagal afferents (not shown). Microglia can be activated by inflammatory stimuli originating in the CNS or by these inflammatory signals from the periphery, and elaborate release of inflammatory mediators in the CNS like cytokines, ROS and nitrogen intermediates, as well as chemokines that further recruit peripheral inflammatory cells to perivascular regions or brain parenchyma. Inflammation also increases neuroactive metabolites from the catabolism of KYN, which is synthesized from Trp by IDO either locally in activated microglia or by macrophages followed by active transport into the brain. Inflammatory cytokines and associated oxidative molecules affect monoamine and glutamate neurotransmission. Panel C. Inflammatory cytokines and the associated release of ROS and nitrogen species can impact neuronal function through several ways including effects on neurotransmitters like monoamines and glutamate. For example, oxidation of BH4, a cofactor required for the synthesis of monoamine precursors, leads to decreased availability and release of monoamines - particularly DA, which requires BH4 for conversion of both Phe to Tyr and Tyr to L-DOPA. Evidence also exists that inflammatory cytokines can decrease expression or function of VMAT2, increase expression and activity of MATs especially the 5-HTT, and reduce expression of MARs like D2R. These effects together lead to a net decrease in synaptic monoamine availability and signaling. Inflammatory and oxidative factors also affect multiple aspects of glutamate transmission particularly by decreasing astrocytic buffering of glutamate by EAAT2, including reversing its efflux while promoting activity of the xCT to increase extracellular glutamate. Increased transport or local production of KYN in the brain and subsequent generation of neurotoxic metabolites like QUIN (a NMDAR agonist) further increase glutamate signaling including at extrasynaptic Rs, which lead to excitotoxicity and downstream generation of ROS (not shown). Peripherally administered acute and chronic inflammatory stimuli, which induce symptoms of depression and anxiety, reliably impact relevant brain regions and circuits in human neuroimaging studies. Panel D. Neuroimaging of patients chronically treated with inflammatory cytokines (e.g., IFN-α) or healthy participants administered stimuli that induce cytokines (e.g., endotoxin, vaccination) have shown that cytokines affect reward and motor-related regions and circuits, as well as those involved in threat, anxiety and emotional processing. For example, PET and MRS studies in IFN-α-treated patients reflect the impact of cytokines on neurotransmitters including reduced striatal DA availability and release as well as increased extracellular glutamate, both of which correlated with reduced motivation and low energy. Corresponding striatal microstructural as well as functional changes have included attenuated VS responses to reward anticipation/receipt and reduced FC between key regions of vmPFC and VS, after acute or chronic administration of IFN-α or other inflammatory stimuli. Inflammatory stimuli also increased neural activation of the amygdala, insula and dACC either independently or together during tasks designed to trigger emotional responses. Importantly, endotoxin also induced greater temporal variance in the amygdala at rest that correlated with lower FC within the SN as well as greater inflammation-induced anxiety. These findings on the impact of exogenous inflammatory stimuli on the brain have established a framework for the growing body of work assessing relationships between endogenous inflammatory markers and structure and function of these regions/circuits in psychiatric patients. Abbreviations: 5-HT - serotonin; 5-HTT - serotonin transporter; ACC - anterior cingulate cortex; BBB - blood-brain barrier; BH4 - tetrahydrobiopterin; CNS - central nervous system; CRP - C-reactive protein; D2R - dopamine 2 receptor; DA - dopamine; dACC - dorsal anterior cingulate cortex; DAMPs - danger-associated molecular patterns; DS - dorsal striatum; EAAT2 - excitatory amino-acid transporter 2; FC - functional connectivity; [18]FDG - fluorodeoxyglucose; [18]FDOPA - fluorodopa; IDO - indoleamine 2, 3-dioxygenase; IFN - interferon; ILs - interleukins; KYN - kynurenine; L-DOPA - levodopa; MAPK- mitogen-activated protein kinase; MARs - monoamine receptors; MATs - monoamine transporters; MRS - magnetic resonance spectroscopy; NE - norepinephrine; NFkB - nuclear factor kappa B; NLRP3 - NOD- LRR- and pyrin domain-containing protein-3; NMDAR - N-methyl-D-aspartate receptor; PET - positron emission tomography; PGE2 - prostaglandin E2; Phe - phenylalanine; QUIN - quinolinic acid; ROS - reactive oxygen species; SN - salience network; TLR - toll-like receptor; STAT - signal transducer and activator of transcription; TNF - tumor necrosis factor; Trp - tryptophan; Tyr - tyrosine; VMAT2 - vesicular monoamine transporter 2; vmPFC - ventromedial prefrontal cortex; VS - ventral striatum; xCT - cystine-glutamate exchanger
