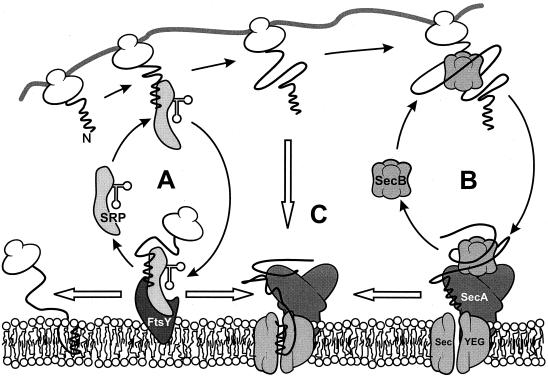
FIG. 3.
Coexisting SRP- and SecB-dependent targeting routes. When a preprotein or integral membrane protein emerges from the ribosome, the signal sequence domain is exposed first. (A) When this domain is highly hydrophobic, it will bind SRP and the nascent preprotein will be targeted to the membrane-bound FtsY at the membrane. At the membrane, SRP is released from the preprotein in a GTP-dependent manner. The preprotein or integral membrane protein is subsequently transferred to the translocase, which consists of the integral membrane proteins SecY, SecE, and SecG and the peripherally bound ATPase SecA, or inserts into the membrane via a different pathway. (B) When the signal sequence escapes SRP binding and the mature domain possesses the right features, SecB will bind the mature domain and targets the preprotein to the membrane-bound SecA. At the membrane, SecB donates the preprotein to SecA. Upon the binding of ATP by SecA, translocation is initiated and SecB is released from the ternary complex. (C) When the preprotein is not recognized by SecB, the signal sequence may target it directly to the membrane-bound SecA.