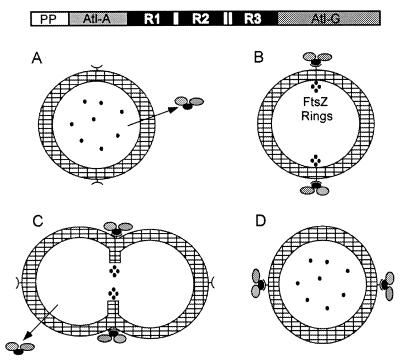
FIG. 20.
Targeting of Atl to the equatorial surface rings of S. aureus. Autolysin is exported from the bacterial cytoplasm (A), and extracellular pro-Atl is targeted to receptor molecules representing the equatorial surface rings of staphylococci that mark the future cell division site (B). Targeting requires the repeats domains (R1 to R3) or pro-Atl. Proteolytic cleavage of pro-Atl generates mature amidase (Atl-A) and glucosaminidase (Atl-G), each of which retains one or two repeat domains and remains bound to the surface rings. The enzymes hydrolyze the cell wall at the cell division site, an event that is probably synchronized with intracellular contraction of FtsZ rings (C), thereby generating two fully separated daughter cells (D). In staphylococci, division occurs perpendicular to the previous cell division plane and targeting of pro-Atl to the second equatorial surface ring can initiate hydrolysis at the future cell division site.