Key Points
Question
Are there disparities in breast cancer treatment and outcomes of patients from sex and gender minority (SGM) groups compared with cisgender heterosexual patients?
Findings
In this exposure-matched case-control study of 92 patients from SGM groups matched to cisgender heterosexual patients by year of breast cancer diagnosis, age, tumor stage, estrogen receptor status, and ERBB2 (HER2) status, those from SGM groups had delays in diagnosis, declined oncologist-recommended therapies more often, and experienced a 3-fold higher rate of breast cancer recurrence compared with cisgender heterosexual patients.
Meaning
Findings suggest several potential health care disparities among patients from SGM groups with breast cancer, necessitating further evaluation to inform interventions.
Abstract
Importance
Sexual orientation and gender identity data are not collected by most hospitals or cancer registries; thus, little is known about the quality of breast cancer treatment for patients from sex and gender minority (SGM) groups.
Objective
To evaluate the quality of breast cancer treatment and recurrence outcomes for patients from SGM groups compared with cisgender heterosexual patients.
Design, Setting, and Participants
Exposure-matched retrospective case-control study of 92 patients from SGM groups treated at an academic medical center from January 1, 2008, to January 1, 2022, matched to cisgender heterosexual patients with breast cancer by year of diagnosis, age, tumor stage, estrogen receptor status, and ERBB2 (HER2) status.
Main Outcomes and Measures
Patient demographic and clinical characteristics, as well as treatment quality, as measured by missed guideline-based breast cancer screening, appropriate referral for genetic counseling and testing, mastectomy vs lumpectomy, receipt of chest reconstruction, adjuvant radiation therapy after lumpectomy, neoadjuvant chemotherapy for stage III disease, antiestrogen therapy for at least 5 years for estrogen receptor–positive disease, ERBB2-directed therapy for ERBB2-positive disease, patient refusal of an oncologist-recommended treatment, time from symptom onset to tissue diagnosis, time from diagnosis to first treatment, and time from breast cancer diagnosis to first recurrence. Results were adjusted for multiple hypothesis testing. Compared with cisgender heterosexual patients, those from SGM groups were hypothesized to have disparities in 1 or more of these quality metrics.
Results
Ninety-two patients from SGM groups were matched to 92 cisgender heterosexual patients (n = 184). The median age at diagnosis for all patients was 49 years (IQR, 43-56 years); 74 were lesbian (80%), 12 were bisexual (13%), and 6 were transgender (6%). Compared with cisgender heterosexual patients, those from SGM groups experienced a delay in time from symptom onset to diagnosis (median time to diagnosis, 34 vs 64 days; multivariable adjusted hazard ratio, 0.65; 95% CI, 0.42-0.99; P = .04), were more likely to decline an oncologist-recommended treatment modality (35 [38%] vs 18 [20%]; multivariable adjusted odds ratio, 2.27; 95% CI, 1.09-4.74; P = .03), and were more likely to experience a breast cancer recurrence (multivariable adjusted hazard ratio, 3.07; 95% CI, 1.56-6.03; P = .001).
Conclusions and Relevance
This study found that among patients with breast cancer, those from SGM groups experienced delayed diagnosis, with faster recurrence at a 3-fold higher rate compared with cisgender heterosexual patients. These results suggest disparities in the care of patients from SGM groups and warrant further study to inform interventions.
This case-control study investigates the quality of breast cancer treatment and recurrence outcomes for patients from sex and gender minority groups compared with cisgender heterosexual patients.
Introduction
Little is known about the quality of breast cancer care among patients from sex and gender minority (SGM) groups. Only 24% of National Cancer Institute Community Oncology Research Program practice groups capture data on sexual orientation, and only 10% capture data on gender identity.1 A 2022 survey of American Society of Clinical Oncology members found that although more than 75% of respondents agreed that knowing sexual orientation and gender identity (SOGI) data is important to providing high-quality care, less than half of them reported collection of SOGI data at their institution.2,3 Even within the SGM literature, women who have sex with women (WSW) and transgender patients are understudied. Of studies funded by the National Institutes of Health that focus on patients from SGM groups, only 13.5% include data on WSW, and just 6.8% include data on transgender patients.4 In the clinic, physicians rarely ask about SOGI status in the context of providing breast cancer care,5 and insufficient sexual history taking is further compounded by the fact that many patients from SGM groups do not reveal their sexual orientation to their care team. Survey estimates of the percentage of WSW who disclose their sexual orientation to their physicians range from 28% to 84%, proportions influenced by a patient’s perception of the safety of the clinic space, the extent to which they disclose their sexual orientation in other circumstances, and certain demographic characteristics (such as patient age, race, and ethnicity).5
Women who have sex with women have a higher theoretical risk of breast cancer because of increased prevalence of risk factors, including nulliparity, alcohol and tobacco use, and higher body mass index.6,7 However, the incidence of breast cancer among WSW—and whether it is higher than that among heterosexual women—remains unclear.7,8,9 Less is known about breast cancer risk for transgender patients, although there is a suggestion of a lower risk of breast cancer among transgender women and transgender men compared with cisgender women.10,11 The 58 000-patient National Health Interview Survey from 2013 to 2017 confirmed for the first time that WSW had lower rates of screening mammography compared with heterosexual patients7,12,13 and that the lower rates of screening mammography exemplified disengagement from primary care. Women who have sex with women were more likely to report difficulty finding a primary care physician with whom they are comfortable, less likely to have consulted a health care professional in the past year, and less likely to have received any form of preventive health care. These differences were independent of reported income or health care insurance status, suggesting that the disparity in preventive care stems from social factors (ie, stigma and difficulty finding a physician with the requisite knowledge to provide high-level primary care to patients from SGM groups) rather than financial ones. This hypothesis is supported by qualitative studies, including cancer research, on patients from SGM groups, that highlight themes of being subjected to systemic homophobia, feeling out of place in heteronormative health care systems, and having to educate their own health care team about caring for SGM patients.14,15,16,17
To our knowledge, there are no data in the literature on treatment quality and patient outcomes for SGM patients with breast cancer. To address the need for data on SGM populations, a 2020 National Academies report called for adding measures of SOGI to ongoing data collection efforts, including clinical settings, and linking data sets that contain SOGI data to existing databases.18 We used an integrated breast cancer research database, Oncoshare, that incorporates electronic medical records (EMRs) of patients treated at Stanford University and the community-based Sutter Medical Network, linked to the statewide California Cancer Registry. We tested the hypothesis that patients from SGM groups with breast cancer experience treatment and outcome disparities compared with matched cisgender heterosexual patients.
Methods
Patient Identification
In this case-control study, a key word search algorithm was used to identify patients from SGM groups treated at Stanford University between January 1, 2008, and January 1, 2022, with an International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) diagnosis of a breast neoplasm and the presence of a SOGI identifier, as has been described previously for cohort identification of SGM patients with cancer.19 eTable 1 in Supplement 1 describes the search terms used to identify patients from SGM groups. This initial cohort identification tool was designed to maximize sensitivity and identified 686 patients’ EMRs that were subsequently manually reviewed, after which the final cohort comprised 92 patients.
Matching Criteria
Using the 92 identified patients from SGM groups, we selected matched cisgender heterosexual patients with breast cancer from the Oncoshare database (n = 184). Oncoshare20,21,22,23 integrates information from the EMRs of 2 San Francisco Bay Area health care systems, Stanford University Health Care and Sutter Medical Network, with patient-level data from the California Cancer Registry, which comprise registries that are also part of the Surveillance, Epidemiology, and End Results Program. Matching variables were age, year of diagnosis, tumor stage, estrogen receptor (ER) status, and ERBB2 (HER2) status. Exact matching was required for tumor stage, ER status, and ERBB2 status (patients with unknown ERBB2 status were allowed to be matched to those with positive, negative, or unknown ERBB2 status). Age was matched within 5 years, and year of diagnosis was matched within 7 years.
Study Measures
After identification of all patients, information was collected from the Stanford University EMR about demographic characteristics (including race and ethnicity, defined by patients), medical history, social history, history of breast cancer screening, breast cancer diagnosis, genetic counseling and testing, breast cancer treatment, clinical trial participation, treatment adherence, and breast cancer recurrence. This study was approved and a waiver for informed consent was granted by the institutional review board at Stanford University. A waiver of authorization was approved by the Stanford University institutional review board, based on the conclusion that disclosure of protected health information involved minimal risk to the participant and the research could not be conducted without access to it. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Statistical Analysis
For the following prespecified quality metrics of the National Comprehensive Cancer Network24,25,26 and American Society of Clinical Oncology,27 we applied both unadjusted and adjusted conditional logistic regression to evaluate the association between each outcome and SGM status, controlling for matched pair: missed guideline-based screening, receipt of appropriate genetic testing, receipt of mastectomy (vs lumpectomy), receipt of chest reconstruction, receipt of adjuvant radiation therapy after lumpectomy, receipt of neoadjuvant chemotherapy for stage III disease, receipt of antiestrogen therapy for at least 5 years for ER-positive disease, receipt of ERBB2-directed therapy for ERBB2-positive disease, and patient refusal of an oncologist’s recommended treatment modality. In addition to SGM status, analyses were adjusted for race and ethnicity, neighborhood-level socioeconomic status,28 and private insurance status. In sensitivity analysis, we further adjusted for tumor stage, age at diagnosis, year of diagnosis, ER status, ERBB2 status, and any variables found to be unbalanced by the exposure or associated with the outcome in bivariate analyses. Odds ratios with 95% CIs and 2-sided Wald tests were reported. All statistical analyses were performed with R, version 4.1 (R Foundation for Statistical Computing). P < .05 was deemed statistically significant.
For analyzing the following 3 time-to-event outcomes, we applied unadjusted and adjusted Cox proportional hazards models stratified by matched pair for time from symptom onset to diagnosis, time from diagnosis to treatment, and time from diagnosis to recurrence. Analyses of residuals were performed to verify the modeling assumptions. Hazard ratios and 95% CIs were reported.
The cohort was described with median values, IQRs, counts, and proportions where appropriate. Differences in baseline demographic and clinical characteristics by SGM status are reported as median differences or differences in proportions with 95% CIs.
Adjusting for Multiple Testing and Sensitivity Analysis
We adjusted for multiple testing by applying the Benjamini-Hochberg correction assuming a false discovery rate of 20%.29 For the findings that remained statistically significant after this correction, we conducted a set of sensitivity analyses; we further adjusted for tumor stage, age at diagnosis, year of diagnosis, ER status, ERBB2 status, and any variables found to be imbalanced by the exposure or associated with the outcome in bivariate analyses. An additional sensitivity analysis was conducted to determine whether race and ethnicity were differential regarding the association between SGM status and the outcomes. Data were analyzed from April 1, 2022, through October 27, 2022.
Results
Patient Characteristics
Demographic characteristics are summarized in Table 1. The median age at diagnosis for all 92 patients was 49 years (IQR, 43-56 years); 74 were lesbians (80%), 12 were bisexual (13%), and 6 were transgender men (6%, including 4 who were heterosexual [4%], 1 who was gay [1%], and 1 who was asexual [1%]). The patients from the SGM group and the cisgender heterosexual patients were not matched by race and ethnicity a priori, and the patients from the SGM group had more non-Hispanic White patients (72 [78.3%] vs 58 [63.0%]; difference, 15.1%; 95% CI, 1.2%-29.3%) and Hispanic patients (13 [14.1%] vs 7 [7.6%]; difference, 6.5%; 95% CI, –3.5% to 16.6%) and fewer Asian or Pacific Islander patients (3 [3.3%] vs 23 [25.0%]; difference, −21.7%; 95% CI, –32.4% to –11.1%) than the cisgender heterosexual patients. No differences in socioeconomic status or insurance type were observed between the 2 groups of patients.
Table 1. Cohort Descriptive and Demographic Characteristicsa.
| Characteristic | Patients, No. (%) | Difference (95% CI)b | |
|---|---|---|---|
| SGM (n = 92) | Cis-Het (n = 92) | ||
| Age at diagnosis, median (IQR), y | 49 (43 to 56) | 49 (43 to 56) | 0.4 (–2.7 to 3.4) |
| Year of diagnosis, median (IQR) | 2014 (2009 to 2017) | 2012 (2009 to 2015) | 0.6 (–1.2 to 2.4) |
| Race and ethnicity | |||
| Latino or Hispanic | 13 (14.1) | 7 (7.6) | 6.5 (–3.5 to 16.6) |
| Non-Hispanic | |||
| Asian or Pacific Islander | 3 (3.3) | 23 (25.0) | –21.7 (–32.4 to –11.1) |
| Black | 2 (2.2) | 3 (3.3) | –1.1 (–6.9 to 4.7) |
| White | 72 (78.3) | 58 (63.0) | 15.1 (1.2 to 29.3) |
| Otherc | 2 (2.2) | 1 (1.1) | 1.1 (–3.7 to 5.8) |
| Neighborhood SESd | |||
| Quintile | |||
| 1 (Lowest SES statewide) | 2 (2.2) | 2 (2.2) | NDe |
| 2 | 16 (17.4) | 13 (14.1) | 3.3 (–8.3 to 14.9) |
| 3 | 16 (17.4) | 17 (18.5) | –1.1 (–13.3 to 11.1) |
| 4 | 19 (20.7) | 22 (23.9) | –3.3 (–16.4 to 9.8) |
| 5 (Highest SES statewide) | 39 (42.4) | 38 (41.3) | 1.1 (–14.3 to 16.4) |
| Private insurance | 61 (66.3) | 67 (72.8) | –6.5 (–20.9 to 7.8) |
| SGM identity or behavior | |||
| Asexual, transgender man | 1 (1.1) | 0 | 1.1 (–2.1 to 4.3) |
| Bisexual, cisgender woman | 12 (13.0) | 0 | 13.0 (5.1 to 21.0) |
| Gay, transgender man | 1 (1.1) | 0 | 1.1 (–2.1 to 4.3) |
| Heterosexual, cisgender woman | 0 | 92 (100) | –100 (–100 to –98.9) |
| Heterosexual, transgender man | 4 (4.3) | 0 | 4.3 (–0.9 to 9.6) |
| Lesbian, cisgender woman | 74 (80.4) | 0 | 80.4 (71.2 to 89.6) |
| Stage | |||
| 0 | 9 (9.8) | 9 (9.8) | NDe |
| I | 32 (34.8) | 32 (34.8) | NDe |
| II | 30 (32.6) | 30 (32.6) | NDe |
| III | 19 (20.7) | 19 (20.7) | NDe |
| IV | 2 (2.2) | 2 (2.2) | NDe |
| Breast cancer receptor status | |||
| ER positive | 77 (83.7) | 77 (83.7) | NDe |
| PR positive | 71 (77.2) | 71 (77.2) | NDe |
| ERBB2 (HER2) positive | 12 (13.0) | 11 (12.0) | 1.1 (–9.6 to 11.7) |
| Triple negative | 13 (14.1) | 13 (14.1) | NDe |
Abbreviations: Cis-Het, cisgender heterosexual; ER, estrogen receptor; ND, no difference; PR, progesterone receptor; SES, socioeconomic status; SGM, sex and gender minority.
Patients matched by age (±5 years) and year of diagnosis (±7 years), tumor stage, ER status, and ERBB2 status.
Median difference; difference in proportions.
Self-identified by patients in their electronic medical record.
Neighborhood SES quintiles were extrapolated from address at diagnosis with US Department of Housing and Urban Development and US Postal Service crosswalk files available through the UCSF Health Atlas.28
Denotes an exact match between SGM and Cis-Het. There was no difference between the 2 groups for these characteristics.
Medical History and Health-Associated Behaviors
Medical history and health-associated behaviors are summarized in eTable 2 in Supplement 1. Rates of diabetes, coronary artery disease, and obesity were similar between the 2 groups of patients. Among the 92 patients from SGM groups compared with cisgender heterosexual patients, there was a suggestion of higher rates of at-risk alcohol use (12 [13.0%] vs 4 [4.3%]; difference, 8.7%; 95% CI, –0.4% to 17.8%)30 and cannabis use (23 [25.0%] vs 7 [7.6%]; difference, 17.4%; 95% CI, 5.9%-28.8%).
Hormonal risk factors are summarized in eTable 3 in Supplement 1. The patient groups were well balanced with respect to median age at menarche, age at first delivery, menopausal status, use of oral contraceptives, and use of hormone replacement therapy. Compared with cisgender heterosexual patients, the 92 patients from SGM groups were more often nulligravid (59 [64%] vs 16 [17%]; difference, 47%; 95% CI, 33%-60%) and had fewer children (median [IQR], 0 [0-0] vs 2 [0-2]).
Breast Cancer Screening and Diagnosis
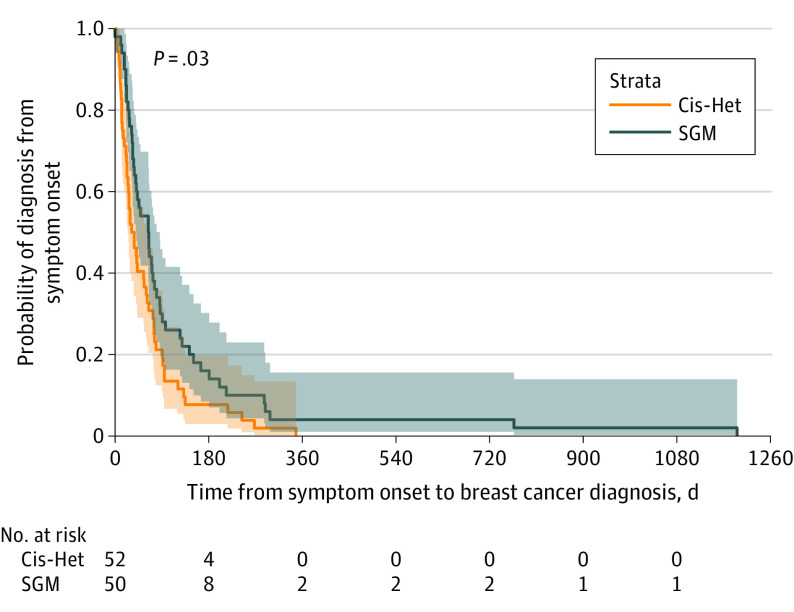
Data summarizing breast cancer screening and diagnosis are presented in Table 2 and eTable 4 in Supplement 1. There was no difference in use of appropriate guideline-based screening mammography between the 2 groups of patients.24,25,26,27 The percentage of patients who presented with symptomatic breast masses was similar between cohorts. However, patients from SGM groups experienced a delay in diagnosis compared with cisgender heterosexual patients, with a median time to diagnosis of 64 days (IQR, 32-118 days) and 34 days (IQR, 16-75 days), respectively. This delay in diagnosis was independent of race and ethnicity, socioeconomic status, or insurance type in multivariable analysis (adjusted hazard ratio, 0.65; 95% CI, 0.42-0.99; P = .04). Figure 1 shows a time-to-event analysis from symptom onset to tissue diagnosis.
Table 2. Adjusted Hazard Ratios or Odds Ratios for Patients From SGM Groups Compared With Cisgender Heterosexual Patients.
| Outcome | Adjusted HR or OR (95% CI)a,b | P value |
|---|---|---|
| Declined oncologist-recommended treatment | 2.27 (1.09-4.47) | .03c |
| Time from symptom onset to diagnosis, d | 0.65 (0.42-0.99) | .04c |
| Time from diagnosis to recurrence, mo | 3.07 (1.56-6.03) | .001c |
| Missed guideline-based screeningd | 1.61 (0.44-5.80) | .47 |
| Appropriate referral to genetic testing | 1.11 (0.71-1.75) | .65 |
| Mastectomy | 1.11 (0.59-2.11) | .74 |
| Chest reconstruction | 0.37 (0.09-1.50) | .17 |
| Adjuvant radiation therapy after lumpectomy | 0.88 (0.41-1.87) | .74 |
| Neoadjuvant chemotherapy for stage III cancer | 1.17 (0.44-3.11) | .76 |
| Antiestrogen therapy for at least 5 y for ER positivity | 0.46 (0.15-1.44) | .18 |
| ERBB2 (HER2) drug for ERBB2 positivity | 0.99 (0.17-5.71) | >.99 |
| Time from diagnosis to treatment, d | 0.96 (0.71-1.31) | .82 |
Abbreviations: ER, estrogen receptor; HR, hazard ratio; OR, odds ratio; SGM, sex and gender minority.
Adjusted by race and ethnicity, socioeconomic status, and private insurance status.
All binary outcomes are reported as ORs. All time-to-event analyses are reported as HRs.
Deemed as significant after the Benjamini-Hochberg correction with a false discovery rate of 20%.
Guideline-based screening in accordance with the National Comprehensive Cancer Network. Patients older than 50 years were assessed for whether they had a screening mammogram within 2 years of their cancer diagnosis, and patients known to carry a pathogenic variant in BRCA1 or BRCA2 before their cancer diagnosis were assessed for whether they had a screening mammogram within 1 year of diagnosis.
Figure 1. Kaplan-Meier Curves of Diagnosis Probability After Symptom Onset.
Patients from SGM groups experienced a delay in diagnosis compared with Cis-Het patients (median time to diagnosis, 64 vs 34 days). Cis-Het indicates cisgender heterosexual; SGM, sex and gender minority.
Genetic counseling and testing and clinical trial enrollment are summarized in eTable 5 in Supplement 1. There were no discrepancies in the rates of appropriate genetic testing referrals or clinical trial engagement (whether that was clinical trial offering, trial enrollment, or type of trial offered or enrolled in).25
Breast Cancer Treatment and Recurrence
Data on breast cancer treatment and cancer recurrence are presented in Table 2 and eTable 6 in Supplement 1. Once patients received a diagnosis, there was no difference in time to first treatment between the 2 patient groups. There was no difference in receipt of lumpectomy vs mastectomy for treatment of localized disease, receipt of adjuvant radiation therapy after lumpectomy, or receipt of neoadjuvant systemic therapy among patients with stage III disease. The point estimate suggested that patients from SGM groups with ER-positive disease may be less likely to receive at least 5 years of antiestrogen treatment compared with cisgender heterosexual patients, although the 95% CIs were wide (adjusted odds ratio, 0.46; 95% CI, 0.15-1.44; P = .18). Reasons for this potential difference included both patient choice and intolerance to therapy. Patients with ERBB2-positive disease received ERBB2-directed therapy at equivalent rates between the 2 patient groups. Patients from SGM groups had a higher rate of documented refusal of an oncologist-recommended cancer-directed therapy (35 [38%] vs 18 [20%]; adjusted odds ratio, 2.27; 95% CI, 1.09-4.74; P = .03), with antiestrogens being the most declined treatment. Patients from SGM groups were also more likely to use alternative medicines compared with cisgender heterosexual patients (42 [46%] vs 28 [30%]; difference, 16%; 95% CI, 0.3%-30.2%).
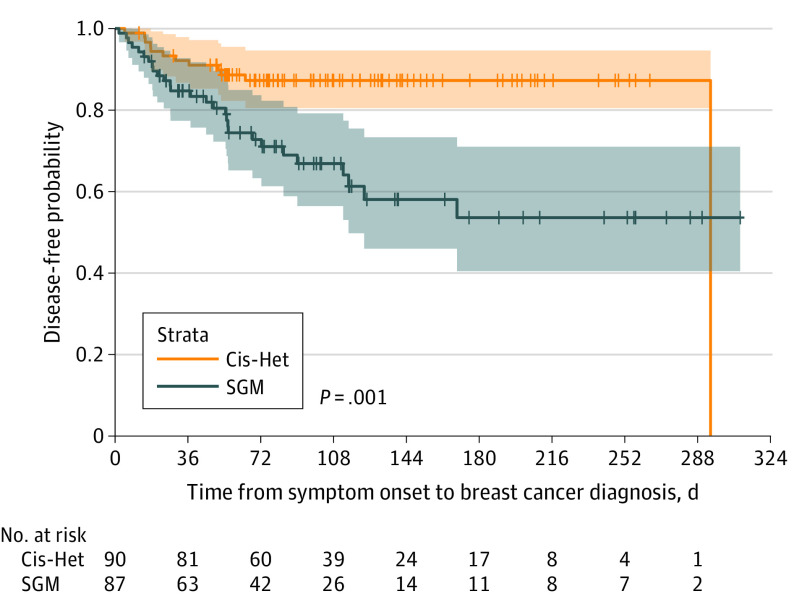
Patients from SGM groups had higher rates of cancer recurrence overall compared with cisgender heterosexual patients (29 [32.2%] vs 12 [13.3%]; difference, 18.9%; 95% CI, 5.8%-32.0%). Two patients in each group had stage IV cancer at diagnosis and so by definition could not have a cancer recurrence. Rates of local cancer recurrence were 17.3% among patients from SGM groups compared with 2.5% among cisgender heterosexual patients, whereas rates of metastatic recurrence were 24.7% among patients from SGM groups compared with 13.6% among cisgender heterosexual patients. Figure 2 presents a Cox proportional hazards model of the rate of recurrence, which shows that patients from SGM groups experienced a 3-fold higher rate than cisgender heterosexual patients, which persisted in multivariable analysis after controlling for race and ethnicity, socioeconomic status, and insurance type (adjusted hazard ratio, 3.07; 95% CI, 1.56-6.03; P = .001).
Figure 2. Kaplan-Meier Curves of Disease-Free Survival After Diagnosis.
Patients from SGM groups experienced a higher rate of breast cancer recurrence compared with Cis-Het patients (32% vs 13%). Cis-Het indicates cisgender heterosexual; SGM, sex and gender minority.
The reported adjusted effect estimates for SGM groups remained significant after accounting for multiple testing using the Benjamini-Hochberg correction with a false discovery rate of 20% (eTable 7 in Supplement 1). Adjustments for factors that were imbalanced between groups, including race and ethnicity, did not affect these estimates for SGM groups significantly in a sensitivity analysis (eTable 8 in Supplement 1).
Discussion
To our knowledge, this is the first study to examine the quality of breast cancer treatment and breast cancer outcomes for patients from SGM groups. In our study, SGM patients had a near doubling of the time from symptom onset to tissue diagnosis, as well as nearly 3 times the recurrence rate compared with matched cisgender heterosexual patients.
Historically, identification of patients from SGM groups has hampered health care disparities research aimed at improving the outcomes of this patient population. This study validates a search term–based method to identify patients from SGM groups in the EMR, but it contrasts with prior studies19,31 in also using behavioral search terms, which resulted in a substantially higher yield than ICD-10 codes and SGM identity categories.32
Health-associated behaviors in patients from SGM groups were consistent with those described in the medical literature. The SGM patients had higher rates of substance use, but apart from having fewer children than cisgender heterosexual patients, they had similar hormonal risk factors (ages at menarche and menopause; oral contraceptive and hormone replacement therapy use).33 These small differences in risk factors do not explain the magnitude of the difference in recurrence rates between the 2 patient groups. In the absence of a clear biological rationale for this difference in outcomes, the reasons for it appear to be associated with structural or social factors.
Patients from SGM groups received breast cancer screening, genetic testing, and clinical trial enrollment at rates similar to those of cisgender heterosexual patients. The equivalent rates of breast cancer screening before breast cancer diagnosis between the 2 patient groups in the present study are unique within the SGM cancer literature; to our knowledge, there is no prior reference for genetic counseling and testing or for clinical trial enrollment. We speculate that these results speak to the well-resourced nature of the population in the San Francisco Bay Area and to the highly motivated and self-advocating nature of patients from SGM groups who have the social capital to know when to disclose their sexual orientation to their medical team.
With respect to diagnostic delay, among patients who presented with a symptomatic breast mass, time from symptom onset to tissue diagnosis was significantly delayed among patients from SGM groups vs cisgender heterosexual patients. The retrospective nature of this study precludes root cause analysis into the causes of this delay; however, within the confines of EMR review, the reasons for the delay appear to stem from both later presentation to care on the part of the patient and longer time to evaluation on the part of the patient’s care team. Clinic-based studies of patients from SGM groups suggest that the reasons for these delays could include patient distrust of health care professionals owing to prior discriminatory experiences or failure on the part of health care professionals to evaluate signs and symptoms reported by SGM patients.34
Patients from SGM groups declined recommended oncologic treatment and used alternative medicine more often than cisgender heterosexual patients; a point estimate was also suggestive of less antiestrogen therapy use by ER-positive SGM patients, although this was not statistically significant. Although we found no association between diagnostic delay and subsequent refusal of oncologist-recommended care among patients from SGM groups, numerous studies underscore that discrimination and mistreatment of SGM patients in health care settings remain common.35,36 Among WSW, those who have previously experienced health care discrimination are less likely to trust health care professionals in the future and often turn to the internet instead of physicians for future health care concerns.37 In addition, patients who use alternative medicine and refuse conventional cancer treatment are known to have worse outcomes, including increased risk of death,38 although given the retrospective nature of this study, it was not possible to establish a causal relationship between alternative medicine use and refusal of conventional cancer treatment. Nevertheless, these findings speak to an opportunity to better align the goals and values of patients from SGM groups with those of their oncologists through targeted education and culturally appropriate supportive care programs.
Overall, this study underscores that patients from SGM groups with breast cancer represent a high-risk population because of delays in diagnosis, noncompliance with recommended therapy, and a higher breast cancer recurrence rate. To address this structural health care disparity in the clinic, physicians must ask about and record the SOGI status of their patients so that they can pay special attention to those from SGM groups, evaluate concerning symptoms quickly, and provide ongoing education to patients about the importance of guideline-based treatment modalities.
Limitations
This study has several limitations. Given the observational and retrospective design, we cannot draw conclusions about causation and note the potential for residual confounding by unknown factors. The 2 patient groups were imbalanced by race and ethnicity, although a sensitivity analysis of race and ethnicity did not demonstrate that this imbalance altered our conclusions; we were unable to evaluate the association of Asian and Pacific Islander status owing to the small number of these patients in the SGM cohort. Sex and gender minority research is always subject to selection bias because patients who choose to disclose their sexual orientation to their care teams and to researchers tend to have greater social resources than those who do not.5 This relative exclusion of the most vulnerable SGM groups from SGM research likely leads to an underestimation of the true extent of disparities affecting the SGM population at large.39 Because this study retrospectively identified patients according to both stated sexual orientation and sexual behavior, those who were not sexually active were less likely to be included; this selection bias is also a known factor that may influence patient decisions to disclose their SGM status to their physician.5 This was a study of patients treated at Stanford University, which serves a high percentage of patients in the top socioeconomic brackets and is located in the San Francisco Bay Area, which tends to be more accepting of people from SGM groups. Although these factors hamper generalizability to all patients from SGM groups with breast cancer, the health care disparities identified here are all the more notable because they persisted despite a more favorable set of social conditions for the SGM population in our hospital’s catchment area.
Conclusions
The results of this case-control study suggest that health care disparities in breast cancer treatment and outcomes of patients from SGM groups should be evaluated by adding SOGI data to large cancer databases and should also be investigated in prospective population-based studies, both of which have the potential to inform health care interventions aimed at improving the quality of care for SGM patients with breast cancer.
eTable 1. Percentage of Patients From SGM Groups With Breast Cancer Identified by Search Term
eTable 2. Medical History of Patients From SGM Groups and Cisgender Heterosexual Breast Cancer Patients
eTable 3. Hormonal Risk Factors of Patients From SGM Groups and Cisgender Heterosexual Breast Cancer Patients
eTable 4. Breast Cancer Screening and Diagnosis of Patients From SGM Groups and Cisgender Heterosexual Breast Cancer Patients
eTable 5. Genetic Testing and Clinical Trial Enrollment of Patients From SGM Groups and Cisgender Heterosexual Breast Cancer Patients
eTable 6. Breast Cancer Treatment and Recurrence of Patients From SGM Groups and Cisgender Heterosexual Breast Cancer Patients
eTable 7. Benjamini-Hochberg Corrected P Values for Prespecified Metrics
eTable 8. Adjusted Association Between Race and Sex and Gender Minority Outcomes
Data Sharing Statement
References
- 1.Cathcart-Rake EJ, Zemla T, Jatoi A, et al. Acquisition of sexual orientation and gender identity data among NCI Community Oncology Research Program practice groups. Cancer. 2019;125(8):1313-1318. doi: 10.1002/cncr.31925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kamen CS, Pratt-Chapman ML, Meersman SC, et al. Sexual orientation and gender identity data collection in oncology practice: findings of an ASCO survey. JCO Oncol Pract. 2022;18(8):e1297-e1305. doi: 10.1200/OP.22.00084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schabath MB, Blackburn CA, Sutter ME, et al. National Survey of Oncologists at National Cancer Institute–designated comprehensive cancer centers: attitudes, knowledge, and practice behaviors about LGBTQ patients with cancer. J Clin Oncol. 2019;37(7):547-558. doi: 10.1200/JCO.18.00551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coulter RWS, Kenst KS, Bowen DJ, Scout. Research funded by the National Institutes of Health on the health of lesbian, gay, bisexual, and transgender populations. Am J Public Health. 2014;104(2):e105-e112. doi: 10.2105/AJPH.2013.301501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boehmer U, Case P. Physicians don’t ask, sometimes patients tell: disclosure of sexual orientation among women with breast carcinoma. Cancer. 2004;101(8):1882-1889. doi: 10.1002/cncr.20563 [DOI] [PubMed] [Google Scholar]
- 6.Clavelle K, King D, Bazzi AR, Fein-Zachary V, Potter J. Breast cancer risk in sexual minority women during routine screening at an urban LGBT health center. Womens Health Issues. 2015;25(4):341-348. doi: 10.1016/j.whi.2015.03.014 [DOI] [PubMed] [Google Scholar]
- 7.Williams AD, Bleicher RJ, Ciocca RM. Breast cancer risk, screening, and prevalence among sexual minority women: an analysis of the National Health Interview Survey. LGBT Health. 2020;7(2):109-118. doi: 10.1089/lgbt.2019.0274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meads C, Moore D. Breast cancer in lesbians and bisexual women: systematic review of incidence, prevalence and risk studies. BMC Public Health. 2013;13(1):1127. doi: 10.1186/1471-2458-13-1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boehmer U, Miao X, Maxwell NI, Ozonoff A. Sexual minority population density and incidence of lung, colorectal and female breast cancer in California. BMJ Open. 2014;4(3):e004461. doi: 10.1136/bmjopen-2013-004461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deutsch MB, Radix A, Wesp L. Breast cancer screening, management, and a review of case study literature in transgender populations. Semin Reprod Med. 2017;35(5):434-441. doi: 10.1055/s-0037-1606103 [DOI] [PubMed] [Google Scholar]
- 11.Jackson SS, Han X, Mao Z, et al. Cancer stage, treatment, and survival among transgender patients in the United States. J Natl Cancer Inst. 2021;113(9):1221-1227. doi: 10.1093/jnci/djab028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McElroy JA, Wintemberg JJ, Williams A. Comparison of lesbian and bisexual women to heterosexual women’s screening prevalence for breast, cervical, and colorectal cancer in Missouri. LGBT Health. 2015;2(2):188-192. doi: 10.1089/lgbt.2014.0119 [DOI] [PubMed] [Google Scholar]
- 13.Agénor M, Pérez AE, Tabaac AR, et al. Sexual orientation identity disparities in mammography among White, Black, and Latina US women. LGBT Health. 2020;7(6):312-320. doi: 10.1089/lgbt.2020.0039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lisy K, Peters MDJ, Schofield P, Jefford M. Experiences and unmet needs of lesbian, gay, and bisexual people with cancer care: a systematic review and meta-synthesis. Psychooncology. 2018;27(6):1480-1489. doi: 10.1002/pon.4674 [DOI] [PubMed] [Google Scholar]
- 15.Pratt-Chapman ML, Alpert AB, Castillo DA. Health outcomes of sexual and gender minorities after cancer: a systematic review. Syst Rev. 2021;10(1):183. doi: 10.1186/s13643-021-01707-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boehmer U, Case P. Sexual minority women’s interactions with breast cancer providers. Women Health. 2006;44(2):41-58. doi: 10.1300/J013v44n02_03 [DOI] [PubMed] [Google Scholar]
- 17.Kent EE, Wheldon CW, Smith AW, Srinivasan S, Geiger AM. Care delivery, patient experiences, and health outcomes among sexual and gender minority patients with cancer and survivors: a scoping review. Cancer. 2019;125(24):4371-4379. doi: 10.1002/cncr.32388 [DOI] [PubMed] [Google Scholar]
- 18.National Academies of Sciences, Engineering, and Medicine. Understanding the Well-Being of LGBTQI+ Populations. National Academies Press; 2020. doi: 10.17226/25877 [DOI] [PubMed] [Google Scholar]
- 19.Burns ZT, Bitterman DS, Perni S, et al. Clinical characteristics, experiences, and outcomes of transgender patients with cancer. JAMA Oncol. 2021;7(1):e205671. doi: 10.1001/jamaoncol.2020.5671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weber S, Seto T, Olson C, Kenkare P, Kurian A, Das A. Oncoshare: lessons learned from building an integrated multi-institutional database for comparative effectiveness research. AMIA Annu Symp Proc. 2012;2012:970-978. [PMC free article] [PubMed]
- 21.Kurian AW, Mitani A, Desai M, et al. Breast cancer treatment across health care systems: linking electronic medical records and state registry data to enable outcomes research. Cancer. 2014;120(1):103-111. doi: 10.1002/cncr.28395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roy M, Purington N, Liu M, Blayney DW, Kurian AW, Schapira L. Limited English proficiency and disparities in health care engagement among patients with breast cancer. JCO Oncol Pract. 2021;17(12):e1837-e1845. doi: 10.1200/OP.20.01093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blayney DW, Seto T, Hoang N, Lindquist C, Kurian AW. Benchmark method for cost computations across health care systems: cost of care per patient per day in breast cancer care. JCO Oncol Pract. 2021;17(10):e1403-e1412. doi: 10.1200/OP.20.00462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gradishar WJ, Moran MS, Abraham J, et al. NCCN Guidelines® insights: breast cancer, version 4.2021. J Natl Compr Canc Netw. 2021;19(5):484-493. doi: 10.6004/jnccn.2021.0023 [DOI] [PubMed] [Google Scholar]
- 25.Daly MB, Pal T, Berry MP, et al. Genetic/familial high-risk assessment: breast, ovarian, and pancreatic, version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19(1):77-102. doi: 10.6004/jnccn.2021.0001 [DOI] [PubMed] [Google Scholar]
- 26.Bevers TB, Helvie M, Bonaccio E, et al. Breast cancer screening and diagnosis, version 3.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16(11):1362-1389. doi: 10.6004/jnccn.2018.0083 [DOI] [PubMed] [Google Scholar]
- 27.Neuss MN, Desch CE, McNiff KK, et al. A process for measuring the quality of cancer care: the Quality Oncology Practice Initiative. J Clin Oncol. 2005;23(25):6233-6239. doi: 10.1200/JCO.2005.05.948 [DOI] [PubMed] [Google Scholar]
- 28.San Francisco School of Medicine Dean’s Office of Population Health and Health Equity . UCSF health atlas. Published 2022. Accessed January 15, 2022. https://www.healthatlas.ucsf.edu/?active=nSES_quintile&geography=counties
- 29.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Stat Soc Ser B Methodol. 1995;57(1):289-300. doi: 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- 30.US Department of Health and Human Services. Helping patients who drink too much: a clinician’s guide. Accessed June 17, 2022. https://pubs.niaaa.nih.gov/publications/Practitioner/CliniciansGuide2005/guide.pdf
- 31.Blosnich JR, Cashy J, Gordon AJ, et al. Using clinician text notes in electronic medical record data to validate transgender-related diagnosis codes. J Am Med Inform Assoc. 2018;25(7):905-908. doi: 10.1093/jamia/ocy022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeglin RJ. The MSM (non)identity: toward understanding sexual behavior and identity in health research and practice with straight men under the umbrella. Sex Res Soc Policy. 2020;17(2):343-352. doi: 10.1007/s13178-019-00398-w [DOI] [Google Scholar]
- 33.Zaritsky E, Dibble SL. Risk factors for reproductive and breast cancers among older lesbians. J Womens Health (Larchmt). 2010;19(1):125-131. doi: 10.1089/jwh.2008.1094 [DOI] [PubMed] [Google Scholar]
- 34.Kamen CS, Alpert A, Margolies L, et al. “Treat us with dignity”: a qualitative study of the experiences and recommendations of lesbian, gay, bisexual, transgender, and queer (LGBTQ) patients with cancer. Support Care Cancer. 2019;27(7):2525-2532. doi: 10.1007/s00520-018-4535-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Herman J, Rankin S, Keisling M, Mottet L, Anafi M. The report of the 2015 Transgender Survey: executive summary. National Center for Transgender Equality. Published December 2016. Updated December 2017. Accessed June 1, 2022. https://transequality.org/sites/default/files/docs/usts/USTS-Executive-Summary-Dec17.pdf
- 36.Eliason MJ, Dibble SL, Robertson PA. Lesbian, gay, bisexual, and transgender (LGBT) physicians’ experiences in the workplace. J Homosex. 2011;58(10):1355-1371. doi: 10.1080/00918369.2011.614902 [DOI] [PubMed] [Google Scholar]
- 37.Johnson MJ, Nemeth LS. Addressing health disparities of lesbian and bisexual women: a grounded theory study. Womens Health Issues. 2014;24(6):635-640. doi: 10.1016/j.whi.2014.08.003 [DOI] [PubMed] [Google Scholar]
- 38.Johnson SB, Park HS, Gross CP, Yu JB. Complementary medicine, refusal of conventional cancer therapy, and survival among patients with curable cancers. JAMA Oncol. 2018;4(10):1375-1381. doi: 10.1001/jamaoncol.2018.2487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Malone J, Snguon S, Dean LT, Adams MA, Poteat T. Breast cancer screening and care among Black sexual minority women: a scoping review of the literature from 1990 to 2017. J Womens Health (Larchmt). 2019;28(12):1650-1660. doi: 10.1089/jwh.2018.7127 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Percentage of Patients From SGM Groups With Breast Cancer Identified by Search Term
eTable 2. Medical History of Patients From SGM Groups and Cisgender Heterosexual Breast Cancer Patients
eTable 3. Hormonal Risk Factors of Patients From SGM Groups and Cisgender Heterosexual Breast Cancer Patients
eTable 4. Breast Cancer Screening and Diagnosis of Patients From SGM Groups and Cisgender Heterosexual Breast Cancer Patients
eTable 5. Genetic Testing and Clinical Trial Enrollment of Patients From SGM Groups and Cisgender Heterosexual Breast Cancer Patients
eTable 6. Breast Cancer Treatment and Recurrence of Patients From SGM Groups and Cisgender Heterosexual Breast Cancer Patients
eTable 7. Benjamini-Hochberg Corrected P Values for Prespecified Metrics
eTable 8. Adjusted Association Between Race and Sex and Gender Minority Outcomes
Data Sharing Statement